Oceanic Institute aims to overcome finfish hatchery bottlenecks

The expansion of the marine aquaculture industry is currently limited, in part, by the inability to successfully bring large numbers of larvae through the critical first-feeding stage. Currently, a program at the Finfish Department of the Oceanic Institute is addressing this challenge by developing methods to culture fish with extremely small larvae that cannot be reared using conventional rotifer- and artemia-based hatchery methods. Included in this group are snappers, groupers and many coral reef species.
Previous projects at the Oceanic Institute were successful in overcoming bottlenecks associated with rearing these small-mouthed larvae by finding a suitable first feed, which ultimately allowed the facility to rear several new species, including the bluefin trevally, an important sport fish in Hawaii; the flame angelfish, a highly valued species in the marine ornamental trade; and the Gulf of Mexico red snapper, a key Gulf species.
The authors achieved this success through the identification of a local calanoid copepod species (P. crassirostris) that has very small and nutritious nauplii.
Calanoid copepods
In addition to facilitating the culture of highly challenging species, the copepods offer several advantages over traditional rotifer and artemia-based technologies. Copepods are thought to be the natural feed source for the larval stages of many fish species. They can be obtained locally, avoiding the use of non-native or invasive species. In addition, their nutritional profile is superior to that of either rotifers or artemia.
However, intensive-production technology for copepods is relatively new and, although successful at smaller scales, required significant development to allow broader application and commercial scale up. Over the past few years, the authors’ work with P. crassirostris focused on refining methods for intensive, pilot-scale production of nauplii with the goal of demonstrating its potential for commercial application.
P. crassirostris potential
Cultures of P. crassirostris originally isolated locally from Kaneohe Bay in Oahu, Hawaii, USA, have been maintained at the Oceanic Institute since 2004. Eggs hatch within seven hours at 25 degrees-C into stage 1 nauplii that are 49 µ wide and 77 µ long, which then take approximately eight days to mature to adulthood at the same temperature. Females are 420 µ long and visually distinct from the smaller 310-µ-long males, both in overall body shape and length of first antennae.
Females produce eggs in clutches of four with healthy females producing up to 28 eggs/day. Females are reproductive throughout their lives, although peak output is generally observed in younger females. The female lifespan can extend to 28 days after hatching. Male P. crassirostris, with no observable mouth parts, generally die within a week of maturing to the adult stage.
Small-scale trial
Early work on P. crassirostris focused on parameters necessary for successful maintenance of stock cultures. The copepods were found to be euryhaline and eurythermal, although cultures are generally maintained in 22 ppt water at 25 degrees-C. Photoperiod studies revealed a wide tolerance to lighting regimens. P. crassirostris are cultured under continuous light from overhead fluorescent fixtures to allow concurrent in situ algae growth.
Algal diets were evaluated in relation to maturation, survival and adult reproduction. Tetraselmis species, Nannochloropsis species, Isochrysis galbana and Chaetoceros muelleri were tested singularly and as combination diets.
Survival to the adult stage increased when nauplii were fed a diet including I. galbana, whereas female fecundity increased with a diet of C. muelleri. Neither Tetraselmis nor Nannochloropsis, either singularly or as mixed diets, led to any noticeable increase in maturation, survival or female reproduction. Therefore, cultures of P. crassirostris are currently maintained on a diet consisting of 150,000 cells/mL of each of the algae I. galbana and C. mulleri.
The authors then focused on nauplii production as a function of population dynamics. In particular, extensive research investigated the relationship between adult copepod density and female fecundity for the purpose of maximizing nauplii production to meet the requirements of a marine fish hatchery.
As has been noted in a wide variety of other copepod species, P. crassirostris reduces reproductive output when population density increases. Although algae density, water quality and cannibalism of young were investigated, only daily harvest and removal of nauplii from culture alleviated density effects on female fecundity in the range of adult densities tested.

Large-scale development
A large-scale nauplii production system was designed and constructed based on the results of earlier small-scale trials. The system consisted of four conical-bottom, 1,500-liter tanks with seven 1,000-liter tanks used for maturation of nauplii to the adult stage.
Each nauplii tank was harvested daily by passive flow through a 105-µ banjo filter into a 20-liter harvest bucket with 38-µ screen panels to retain eggs and nauplii but allow the flow of culture water out of the harvest bucket. Water was then pumped back into the main nauplii production tank at 30 L/minute to complete the circuit. Ninety-five percent of nauplii and eggs can be removed from the production tank with little effort in four hours.
Every day, eggs and nauplii from the harvest are stocked into a clean maturation tank at 20/mL, while a maturation tank is harvested for adults to stock into the nauplii production tank to maintain female densities of 1-2/mL. In addition, each nauplii production and adult maturation tank receives 150,000 cells/mL of both I. galbana and C. mulleri, resulting in a daily need of 1.95 trillion cells of each alga for the entire system, which are produced on site in the algae production laboratory.
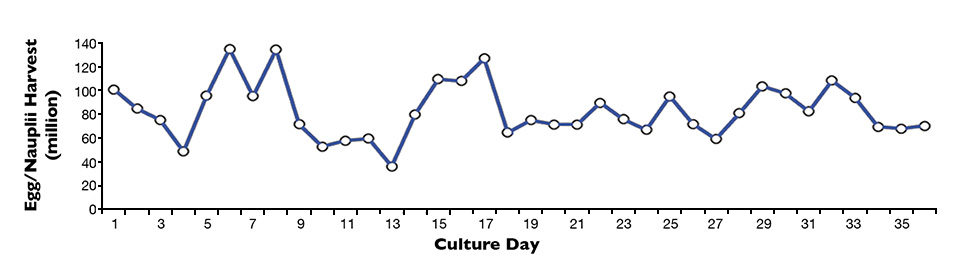
Harvest figures recorded over 36 days for eggs and nauplii from four nauplii production tanks are shown in Fig. 1. Daily harvests ranged from 36 million to 135 million eggs and nauplii, with an average of 83 million eggs and nauplii.
The greatest variation in harvest yield was due to algae culture shortages, which led to subsequent nauplii production declines in the system. The inadequate supply of algae for the nauplii production tanks resulted in an immediate reduction in eggs and nauplii produced.
Normal production values returned after several days of properly sustained feed densities. However, if algae production could not be stabilized, a subsequent lack of algae for the maturation tanks led to a reduction in the quantity of adults used to replace deceased females in nauplii production tanks. Having fewer females further reduced egg and nauplii harvests.
Perspectives
Future research on copepod production at the Oceanic Institute will focus on further refinement of the intensive nauplii production system – given the promising preliminary data showing more than 100 million eggs and nauplii harvested daily. Specifically, it would be desirable to develop more efficient algae culture methods or, ideally, an appropriate live microalgae substitute.
Although this system shows exciting potential for supplying large numbers of copepod nauplii, the expense of maintain-
ing and operating an algae production facility to support it represents a large portion of the total cost. Therefore, future efforts will continue to address these important challenges.
(Editor’s Note: This article was originally published in the January/February 2014 print edition of the Global Aquaculture Advocate.)
Authors
-
M. Dean Kline
Finfish Department
Oceanic Institute
41-202 Kalanianaole Highway
Waimanalo, Hawaii 96795 USA -
Chatham K. Callan, Ph.D.
Finfish Department
Oceanic Institute
41-202 Kalanianaole Highway
Waimanalo, Hawaii 96795 USA -
Charles W. Laidley, Ph.D.
Finfish Department
Oceanic Institute
41-202 Kalanianaole Highway
Waimanalo, Hawaii 96795 USA
Tagged With
Related Posts

Innovation & Investment
Artemia, the ‘magic powder’ fueling a multi-billion-dollar industry
Artemia, microscopic brine shrimp used as feed in hatcheries, are the unsung heroes of aquaculture. Experts say artemia is still inspiring innovation more than 50 years after initial commercialization. These creatures are much more than Sea-Monkeys.

Health & Welfare
Acclimating shrimp postlarvae before pond stocking
Shrimp postlarvae acclimation before stocking into the various growout systems (ponds, raceways, tanks) is a critical – and often overlooked, sometimes taken for granted – step in the shrimp culture process. Various water quality parameters should be changed slowly so that the young shrimp have the time to gradually adapt to the new conditions.

Health & Welfare
Aquamimicry: A revolutionary concept for shrimp farming
Aquamimicry simulates natural, estuarine production conditions by creating zooplankton blooms as supplemental nutrition to the cultured shrimp, and beneficial bacteria to maintain water quality. Better-quality shrimp can be produced at lower cost and in a more sustainable manner.

Health & Welfare
A holistic management approach to EMS
Early Mortality Syndrome has devastated farmed shrimp in Asia and Latin America. With better understanding of the pathogen and the development and improvement of novel strategies, shrimp farmers are now able to better manage the disease.


