Proper postlarval nutrition, controlling microflora lead to higher quality hatchery output

Shrimp postlarvae quality significantly contributes to higher and more sustainable shrimp production. A variety of factors – including nutrition, treatment of disease, and control of microflora – contribute to seedstock health in optimal hatchery operations.
Direct nutritional effects on postlarval quality
The nutritional value of artemia spp. nauplii, still the major food organism used for culturing shrimp postlarvae (PL), is highly variable depending on geographic origin, especially in their content of the essential fatty acid eicosapentaenoic acid 20:5 omega-3 (EPA). However, the biochemical composition of artemia can easily be manipulated through the bioencapsulation technique using emulsified marine oils containing high levels of EPA and DHA (docosahexaenoic acid 22:6 omega-3).
Lavens, Mortality rates of P. monodon postlarvae, Table 1
| Postlarval Stage | Salinity Stress Condition | Mortality 24 h After Challenge (%) Low-HUFA Diet | Mortality 24 h After Challenge (%) Medium-HUFA Diet | Mortality 24 h After Challenge (%) High-HUFA Diet | Reference |
|---|
Postlarval Stage | Salinity Stress Condition | Mortality 24 h After Challenge (%) Low-HUFA Diet | Mortality 24 h After Challenge (%) Medium-HUFA Diet | Mortality 24 h After Challenge (%) High-HUFA Diet | Reference |
|---|---|---|---|---|---|
| PL10 | 1 h at 7 g/l | 82a (6) | – | 15b (5) | Tackaert et al., 1992 |
| PL10 | 2 h at 10 g/l | 75a (2) | 35b (15) | 10c (2) | Rees et al., 1994 |
| PL15 | 1 h at 10 g/l | 73a (7) | – | 13b (4) | Kontara et al., 1995a |
HUFA-enriched artemia
Several authors have reported that feeding highly unsaturated fatty acid (HUFA)-enriched artemia to Penaeus monodon PLs resulted in improved PL quality, expressed as their ability to survive an exposure to a salinity shock (Table 1). Other research has documented that very high dietary levels of 31 milligrams per gram DW HUFA (DHA/EPA ratio of 0.5) did not improve postlarval stress resistance, suggesting that an excessive supply of HUFA may not be beneficial to the shrimp.
Phosphatidyl choline
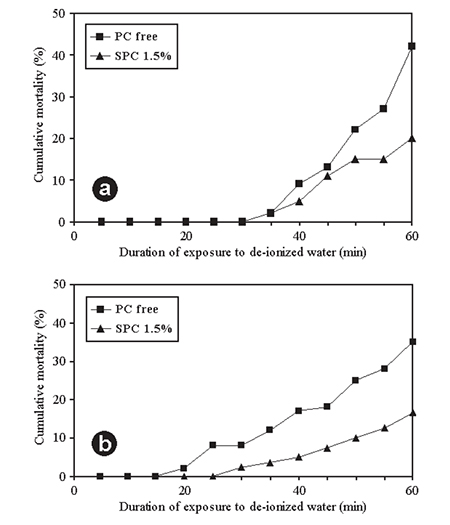
Other lipid components, such as phosphatidyl choline (PC), may have similar effects on P. japonicus and Litopenaeus vannamei PLs. For both species, 1.5 percent inclusion of soybean PC in the diet resulted in a significant increase in stress resistance, expressed as mortality after exposure for increased time intervals to deionized water (Fig. 1).
Vitamins
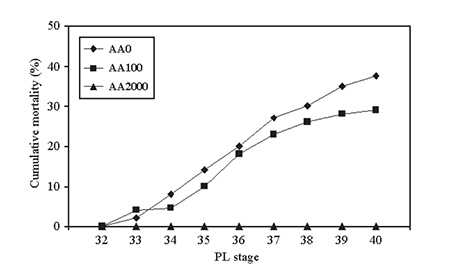
Vitamins may also play an important role in improving the quality of hatchery-produced PLs. Culture tests with vitamin C-boosted microbound diets for P. monodon and L. vannamei PLs confirmed the hypothesis that dietary ascorbic acid (AA) improved overall physiological condition in postlarvae (Table 2).
Optimal resistance to a Vibrio harveyi infection was observed when L. vannamei PLs were fed a megadose (2,000 mg AA equivalent per kilogram) of vitamin C (Fig. 2). An increase from 230 to 810 milligrams per kilogram in astaxanthin levels in the diet of P. monodon also resulted in a significant drop of the stress index (cumulative mortality index during an osmotic shock from 56 to 33).
Lavens, Mortality rates of shrimp postlarvae fed microbound diets, Table 2
| Postlarval Stage | Salinity Stress Condition | Mortality 24 h After Challenge (%) 0 mg/kg VitC* in Diet | Mortality 24 h After Challenge (%) 100 mg/kg VitC* in Diet | Mortality 24 h After Challenge (%) 2000 mg/kg VitC* in Diet | Reference |
|---|
Postlarval Stage | Salinity Stress Condition | Mortality 24 h After Challenge (%) 0 mg/kg VitC* in Diet | Mortality 24 h After Challenge (%) 100 mg/kg VitC* in Diet | Mortality 24 h After Challenge (%) 2000 mg/kg VitC* in Diet | Reference |
|---|---|---|---|---|---|
| L. vannamei, PL40 | 1 h at 0 g/l | 67a (10) | 37b (6) | 10c (8) | Kontara et al. 1997 |
| L. vannamei, PL36 | 1 h at 8 g/l | 95a (1) | 95a (1) | 94a (1) | Lavens et al., 1998 |
| P. monodon, PL40 | 2 h at 0 g/l | – | 83a (2) | 56b (7) | Merchie et al., 1998 |
| P. monodon, PL25 | 1 h at 0 g/l | 91a (3) | 91a (4) | 94b (4) | Lavens et al., unpubl. |
Values within the same row with the same superscript are not significant different (P>0.05); n = 3.
Table 2. Mortality rates of shrimp postlarvae fed microbound diets with different levels of vitamin C, after challenge to an osmotic stress test.
Effect of immunostimulants on postlarval quality
Immunostimulants have gained interest as a valuable alternative to antibiotics and vaccines in the fight against infectious diseases in shrimp farming. A number of commercial products (yeast preparations, beta-glucans, polysaccharides) are now available, all claiming to have positive effects on disease resistance. Recent experiments run with specific immunostimulants are referenced below.
Commercial booster diet
A commercial booster (Dry Immune Selco®; DIS, Inve Aquaculture NV), containing a mix of immunostimulants and vitamins C and E, was developed for bioencapsulation in artemia. P. monodon PL11 were fed non-enriched (control) or DIS-enriched artemia for five days. Twenty-four hours after the start of the experiment, the DIS group suffered significantly lower mortality (20 percent vs. 90 percent) than the control group when the animals were submitted to a salinity stress test (1 hr at 0 ppt). Similar results were reported for P. indicus PL16.
Microparticulate diet
A microparticulate diet (Stresspak, Inve) containing high levels of selected micronutrients in combination with immunostimulants was developed as a partial replacement for live food, to fortify shrimp PLs during transportation and just after stocking in ponds. Handling conditions at these times are considered very stressful and may normally result in a substantial PL mortality. A 50 percent supplementation of this diet for 14 days from PL1 stage onwards resulted in 30 percent higher survival after subsequent 12-h transportation to the nursery.
Better control of microflora during hatchery rearing
The live food used in larviculture operations is an important source of microbial contamination. For example, artemia nauplii are heavily contaminated with bacteria of more than 107 colony forming units (CFU) per gram, mostly Vibrio spp. These bacteria may be potentially pathogenic, or may stress the shrimp PL and make them more susceptible to viral infections.
Several reports have shown that disinfection – and even decapsulation – of the cysts prior to hatching incubation does not prevent bacteria from developing during the final hours before harvesting of the nauplii. Furthermore, thorough washing of the hatched nauplii does not have a significant effect on microbial contamination levels.
Reducing bacterial numbers
Application of the recently commercialized DC Artemia cysts® (Inve) resulted in a drastic reduction of bacterial numbers in hatching water all the way through the hatching incubation of the cysts. At harvest of the nauplii, bacterial levels reached 107 CFU per milliliter (total counts, use of Marine Agar: M.A.) in control tanks versus 103 CFU per milliliter in the DC treatment. Vibrio levels (specific counts, Thiosulphate-Citrate- Bile-Sucrose agar: TCBS) were 106 CFU per milliliter versus 102 CFU per milliliter .
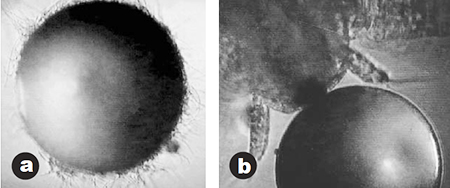
A comparable pattern was observed with regard to bacterial load of the nauplii itself. Nauplii hatched out of DC cysts contained 10,000 times less bacteria (both on MA and TCBS) than the control. Microscopic examination of the cysts 24 hours after incubation revealed the presence of filamentous bacteria, protozoa, and other epizoics on the cyst surface of the control group only (Fig. 3). Similarly, after 24-hour enrichment of the nauplii with Selco® versus DC Selco® (Inve), bacterial contamination levels were 108, 105 CFU per milliliter for total counts, and 108 and 104 CFU per milliliter for Vibrio.
Optimizing control over microflora
Control over microflora in a shrimp hatchery can be further optimized through improved hygiene and the use of modular units that strictly separate hatchery sections. The adoption of regular shutdowns and sterilization periods between production cycles, use of probiotics, and minimal use of antibiotics are also effective measures.
Antibiotics
In recent years, several hatchery operators (especially in Latin America) have been inoculating larviculture tanks with selected Vibrio species with so-called probiotic effects. This empirical development certainly has great potential, but a more thorough scientific approach is required to develop well-documented and effective methods for microbial management in larviculture systems.
The use of antibiotics should be disapproved, certainly for prophylactic, but even for therapeutic treatment. In the latter case, it should be restricted to oral treatment only – through the application of the bioencapsulation technique with artemia, for example.
Conclusion
Research continues to improve larval shrimp diets through better dietary regimes, use of immunostimulants, and better control of microflora. These improve the production of predictable supplies of high-quality seedstock to support shrimp farming activities.
Note: Cited references available on request from the authors.
(Editor’s Note: This article was originally published in the December 2000 print edition of the Global Aquaculture Advocate.)
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Authors
-
Patrick Lavens, Ph.D.
Laboratory of Aquaculture & Artemia Reference Center
University of Gent
Gent, Belgium -
Patrick Sorgeloos, Ph.D.
Laboratory of Aquaculture & Artemia Reference Center
University of Gent
Gent, Belgium
Tagged With
Related Posts

Aquafeeds
A look at phospholipids in aquafeeds
Phospholipids are the major constituents of cell membranes and are vital to the normal function of every cell and organ. The inclusion of phospholipids in aquafeeds ensures increased growth, better survival and stress resistance, and prevention of skeletal deformities of larval and juvenile stages of fish and shellfish species.

Health & Welfare
Blue alternative: High Health introduces SPF blue shrimp to Thailand
Blue shrimp are very similar to Pacific white shrimp, and can be raised under similar conditions. Blues grow faster and tolerate lower water temperatures.

Health & Welfare
Brazil’s intensive shrimp nursery systems improve PL management, grow-out ponds
Intensive nursery systems are an extension of hatcheries to acclimate postlarvae to farm conditions and assess quality and health prior to pond stocking.

Responsibility
Growth away from the coast: Examining inland shrimp farming
Inland shrimp culture has numerous advantages – improved biosecurity, lower cost of land and reduced conflicts with other users of common resources like land and water – and will continue to expand into new areas.


