Quality control ranges have been established for oxytetracycline and nine other antimicrobial agents
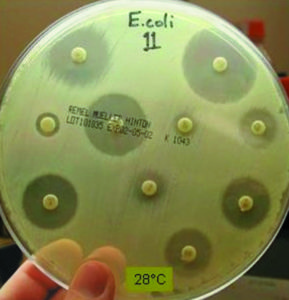
Until recently, researchers lacked standardized methods to accurately monitor changes in the susceptibility of and development of resistance in aquatic bacterial pathogens to antimicrobial agents used in global aquaculture. The many variations in testing methods used by aquatic disease specialists have led to inconsistent results that make correlation between laboratories difficult.
Working group studies
To address such issues, the National Committee for Clinical Laboratory Standards (NCCLS) created the Subcommittee on Veterinary Antimicrobial Susceptibility Testing – Aquaculture Working Group in 1998. Recently renamed Clinical and Laboratory Standards Institute (CLSI), NCCLS charged the group to produce guidance documents for methods of testing the antimicrobial susceptibility of bacteria isolated from the aquatic environment.
Members of this working group have since identified suitable quality control strains and coordinated two multiple-laboratory studies that included researchers from the United States, Denmark, Norway, Greece, Australia, and Canada. They standardized the agar disk diffusion and broth microdilution testing methods. This work defined zones of inhibition and minimum inhibitory concentration quality control ranges for two bacterial strains: Escherichia coli ATCC 25922 and Aeromonas salmonicida subsp. salmonicida ATCC 33658.
Control ranges
These studies represent the first published reports conducted in accordance with approved NCCLS guidelines to establish quality control ranges at temperatures lower than 35 degrees-C for antimicrobial susceptibility testing of aquatic bacteria. The standardized methods and approved ranges should constitute a foundation for the establishment of ranges for other antimicrobial agents important to the aquaculture industry and additional pathogen-testing methods. The methods and associated quality control ranges will likely be used to develop susceptible, intermediate and resistant breakpoints for antimicrobial agents used in aquaculture.
The standardized agar disk diffusion methods were published by NCCLS in 2003 in a report titled “Methods for Antimicrobial Disk Susceptibility Testing of Bacteria Isolated From Aquatic Animals.” A more detailed description of this work can be found in a 2003 article by Miller and coauthors in the Journal of Clinical Microbiology. The broth microdilution methods will be published in a CLSI report to be published next year titled “Methods for Broth Dilution Susceptibility Testing of Bacteria Isolated From Aquatic Animals.”
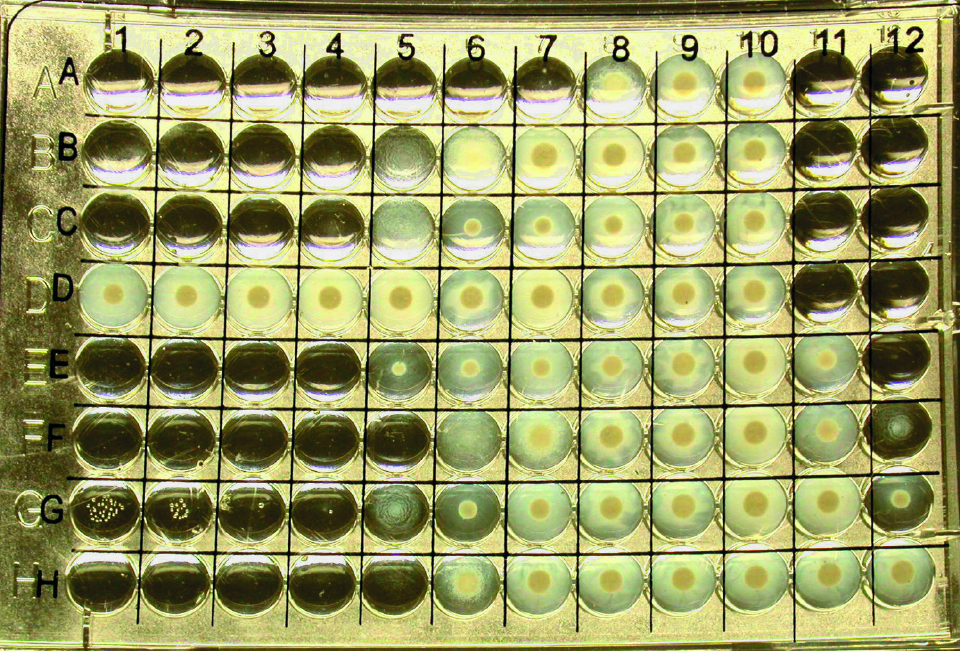
Continued development
CLSI has gained international support for its antimicrobial susceptibility testing methods, with virtually all hospitals in the USA and many overseas facilities closely following the methods and quality control criteria outlined in its documents. An increase in worldwide use of the documents, coupled with the many antimicrobial agents approved for use in aquaculture, has steered the Aquaculture Working Group toward continued development of standardized antimicrobial susceptibility tests.
Thus far, quality control ranges have been established for oxytetracycline, ormetoprim-sulfadimethoxine, oxolinic acid, florfenicol, flumequine, erythromycin, ampicillin, enrofloxacin, gentamicin and trimethoprim-sulfamethoxazole when testing at 22, 28 and 35 degrees-C.
(Editor’s Note: This article was originally published in the December 2004 print edition of the Global Aquaculture Advocate.)
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Authors
-
Ron A. Miller, M.S.
U.S. Food and Drug Administration
Center for Veterinary Medicine
Office of Research
8401 Muirkirk Road
Laurel, Maryland 20708 USA[118,111,103,46,97,100,102,46,109,118,99,64,49,114,101,108,108,105,109,114]
-
Robert D. Walker, Ph.D.
U.S. Food and Drug Administration
Center for Veterinary Medicine
Office of Research
8401 Muirkirk Road
Laurel, Maryland 20708 USA -
Renate Reimschuessel, Ph.D., VMD
U.S. Food and Drug Administration
Center for Veterinary Medicine
Office of Research
8401 Muirkirk Road
Laurel, Maryland 20708 USA
Tagged With
Related Posts

Health & Welfare
A comprehensive look at the Proficiency Test for farmed shrimp
The University of Arizona Aquaculture Pathology Laboratory has carried out the Proficiency Test (PT) since 2005, with 300-plus diagnostic laboratories participating while improving their capabilities in the diagnosis of several shrimp pathogens.

Health & Welfare
Antibiotic-resistant bacteria, part 1
No antimicrobial agent has been developed specifically for aquaculture applications. However, some antibiotic products used to treat humans or land-based animals have been approved for use at aquaculture facilities.

Intelligence
Non-compliance on EU antibiotics order may cost India shrimp industry
India, now the world’s top farmed shrimp exporter, may lose access to the European marketplace by 2018 due to ongoing issues with antibiotics.

Responsibility
Phage therapy provides targeted bacteria treatment
The use of phages is an alternative to antibiotics in the control of pathogenic bacteria. Phage therapy offers low-cost, low-toxicity treatment and quick bactericidal effects.


