Industry impacted by recurrent disease outbreaks in major estuaries

The Sydney rock oyster (Saccostrea glomerata) is a commercially important species mainly cultivated in estuaries along the east coast of Australia. Production of this species began in the mid-1800s and has since grown to become the largest aquaculture industry in New South Wales. Sydney rock oyster sales generate approximately AUS $36 million annually.
Industry challenges
Since the mid-1970s, Australia’s rock oyster industry has suffered decreasing production attributed to a combination of factors that include competition from other species, disease and declining water quality. The industry is significantly impacted by recurrent Queensland unknown (QX) disease outbreaks caused by Marteilia sydneyi in major oyster-growing estuaries.
The New South Wales Department of Primary Industries commenced a breeding program at the Port Stephens Fisheries Centre in 1990 to address the challenges faced by growers. It recently produced a fifth generation of oysters resistant to QX and winter mortality disease, and a sixth generation of faster-growing oysters.
New rearing protocols
Unfortunately, the promising results from the breeding program have not been fully adopted because of persistent hatchery or nursery problems that cause mortalities over 80 percent at either the early larval stage or 7 to 45 days following settlement.
A Fisheries Research and Development Corporation project therefore began in 2003 to develop new rearing protocols and techniques. Early results have increased the survival of larvae and spat, and improved growth rates and hatchery productivity.
Under the umbrella of this larger project, the toxicity of substances commonly used at the Port Stephens Fisheries Centre bivalve hatchery and the effects of handling procedures on oyster development were tested. The main aims were to identify substances and procedures that posed a risk to larval health, and evaluate new materials and procedures, as well.
Toxicity study
A broad range of substances commonly used for sterilization, cleaning, and other routine purposes in bivalve, fish and crustacean hatcheries were examined (Table 1). These tests were also used to target other sources of contamination for larval tanks and the stored rain water and bore water used in the dilution of sea water and cleaning. The chronic toxic effects of each substance were determined and ranked by quantifying embryo-larval development after 48 hours of exposure.
Dove, Hatchery substances and procedures included in initial testing, Table 1
| Substance/Procedure | Application | Risk to Larvae* |
|---|---|---|
| Sodium hypochlorite (chlorine) | Sterilization of tanks, plumbing, equipment, clothes, and sea water | High |
| Iodine (iodophors) | Sterilization of external surfaces of bivalve broodstock, footwear, equipment, hands, and laboratory surfaces | Low |
| Potassium | Sterilization of equipment | Low |
| Virucidal disinfectant | Sterilization and cleaning of tanks | High |
| Cleaning and sanitizing agent | Sterilization and cleaning of tanks and equipment | Low |
| Rain water | Cleaning, rinsing, and dilution of sea water for salinity reduction | High |
| Bore water | Cleaning, rinsing, and dilution of sea water for salinity reduction | High |
| Algal supernatant | Food for larvae | Unknown |
| Egg screening | Egg screening to rinse, separate, or remove feces and debris from spawning | Unknown |
* Under present practices at the Port Stephens Fisheries Centre bivalve hatchery.
Substances that through accidental contamination could easily come in contact with rearing tanks at potentially toxic concentrations were classified as high-risk and subjected to more rigorous ecotoxicological testing. Data from the range-finding tests was also used to calculate the concentrations of substances to use in subsequent tests.
Chlorine, the virucidal disinfectant, bore water, rain water and algal supernatant were selected for further testing based on the results from the first experiment and their regular use in the early stages of larval rearing in the bivalve hatchery.
Virucidal disinfectant
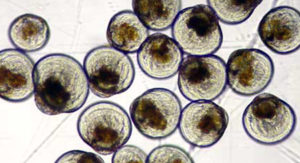
The virucidal disinfectant was composed of inorganic peroxygen compound, inorganic salts, organic acid, anionic detergent, fragrance and dye. Concentrations of virucidal disinfectant in the 0.05 to 0.50 milligrams per liter range were found to significantly inhibit the development rates of rock oyster embryos. Furthermore, this substance could easily contaminate incubation tanks, as it was frequently used to clean and disinfect tanks.
Approximately 500 ml of virucidal disinfectant were applied per 1,000-liter tank at a concentration of 5 grams per liter before fertilized eggs were added and between regular water changes during larval rearing. If the tanks were not rinsed, the concentration of virucidal disinfectant could be as high as 2.5 milligrams per liter, which could prevent more than 80 percent of the oyster embryos from developing to the D-veliger stage.
Chlorine
Applied as sodium hypochlorite, chlorine was used in the hatchery at 20 milligrams per liter to sterilize tanks, plumbing, equipment, and sea water. The chlorine applied to tanks and equipment was generally rinsed off and not neutralized with thiosulphate before use.
Toxicity tests revealed that chlorine at concentrations of 0.83 to 1.66 kilograms per liter caused significant abnormal development in oyster larvae. Therefore, it was critical to ensure that equipment treated with chlorine, such as buckets and screens used to handle larvae, was thoroughly rinsed before it came into contact with eggs, embryos or larvae. Great care must be exercised when handling chlorine in hatchery areas where oysters spawn and larvae are incubated.
Rain water, bore water
Other notable findings were that rain water and bore water caused abnormal development at concentrations of 1 and 10 percent, respectively. Experiments requiring the dilution of sea water with rain or bore water were regularly conducted at the hatchery. Treatments that used deionized water in the same concentrations as rain water and bore water did not significantly affect embryonic development. Hence, the abnormal development in treatments that contained rain water and bore water could be attributed to contaminants in those waters and not osmotic effects caused by the decreased salinity levels.
Algal cultures can contain metabolites that impact larval health or become bacterized. Tests showed that algal supernatant obtained added at the regular feed dose rate did not inhibit larval development.
Handling procedures
The effects of screening fertilized eggs were examined to ensure the process did not physically damage the eggs. Eggs are typically screened through a 65-µ screen to remove any feces and debris accumulated during spawning.
Repeated screening of eggs up to 16 times had no significant effect on embryonic development. Further handling experiments are planned to investigate the effects on early larval development of temperature shocks that can occur following spawning.
(Editor’s Note: This article was originally published in the August 2005 print edition of the Global Aquaculture Advocate.)
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Authors
-
Michael C. Dove, Ph.D.
NSW Department of Primary Industries
Port Stephens Fisheries Centre
Taylors Beach, NSW 2316 Australia[117,97,46,118,111,103,46,119,115,110,46,115,101,105,114,101,104,115,105,102,64,109,101,118,111,100]
-
Wayne A. O’Connor, Ph.D.
NSW Department of Primary Industries
Port Stephens Fisheries Centre
Taylors Beach, NSW 2316 Australia
Tagged With
Related Posts

Aquafeeds
Live diets for larval fish, shrimp: Production, enrichment, feeding strategies
Live diets for reared marine larvae must be cost-effective and versatile while providing good nutrition and being easily captured and digested. Copepods offer superior nutritional value, but their rearing requires space and is laborious.

Responsibility
Ailing waterways hail the oyster’s return
The Lower Hudson Estuary and Chesapeake Bay, two waterways once home to thriving oyster beds, would welcome the shellfish’s return. Aquaculture initiatives in both areas aim to reinvigorate the water and the communities they support.

Responsibility
A helping hand to lend: UK aquaculture seeks to broaden its horizons
Aquaculture is an essential contributor to the world food security challenge, and every stakeholder has a role to play in the sector’s evolution, delegates were told at the recent Aquaculture’s Global Outlook: Embracing Internationality seminar in Edinburgh, Scotland.

Innovation & Investment
Australia incentivizing innovation in aquaculture
A government-funded $3 million (AUS) project by innovationXchange, WWF and others seeks transformative solutions from entrepreneurs for small-scale producers in the Indo-Pacific region. The competition’s three challenges include fresh thinking on feed, “new ocean products” and sustainable design.


