Control of pathogen entry and proliferation an essential aspect of animal production

With the emergence of marine finfish aquaculture in the United States, there is a growing need to improve the biosecurity of marine hatchery operations, whether targeting fingerling production for onshore or open ocean grow-out. The control of pathogen entry and proliferation is an essential aspect of intensive animal production that is rapidly becoming one of the more difficult challenges facing the finfish culture industry worldwide.
The aquatic nature of aquaculture operations and frequent import of broodstock, eggs and live feeds create many opportunities for pathogen entry into hatchery operations. Once imported, pathogens can rapidly proliferate, particularly in the warmwater hatchery operations of the state of Hawaii and throughout the Pacific.
Moi fingerling production
After the success of its open ocean cage demonstration project, Oceanic Institute began culturing moi fingerlings for a commercial cage start-up in collaboration with Grove Farms Moi & Poi, under funding from National Oceanic and Atmospheric Administration Marine Aquaculture Program.
To make this venture economically viable, fingerling production was scaled up from less than 100,000 to over 250,000 fingerlings per run, and operations expanded to a monthly basis. Note that moi spawn on a monthly lunar cycle and require only 25 days in the hatchery, effectively allowing up to 12 production cycles/year.
Pathogen pathways

Soon after reaching target output, the operation began encountering larval mortality issues that led to run terminations soon after larvae began feeding on rotifers. Preliminary diagnostics implicating the presence of pathogenic bacteria during feeding initiation and gut colonization led the authors to begin examining the vectors for pathogen entry, movement and proliferation within the marine warmwater hatchery environment.
Initial screens revealed a wide variety of bacteria – some of which were pathogenic – entering the hatchery operations through eggs, water and live feeds. Vertical pathogen transfer through eggs can be highly problematic in that egg introductions from broodstock holding systems to the hatchery are clearly necessary to stock tanks.
Earlier work at Oceanic Institute revealed a number of highly pathogenic bacterial strains associated with moi eggs. This work also showed that a rigorous “rinsing” procedure with sterilized seawater proved as effective as the use of specific egg disinfectants. Subsequent studies indicated that iodine and hydrogen disinfectants tended to be ineffective at lower concentrations, and at higher concentrations are detrimental to the small eggs of many pelagic spawning species.
Waterborne bacteria
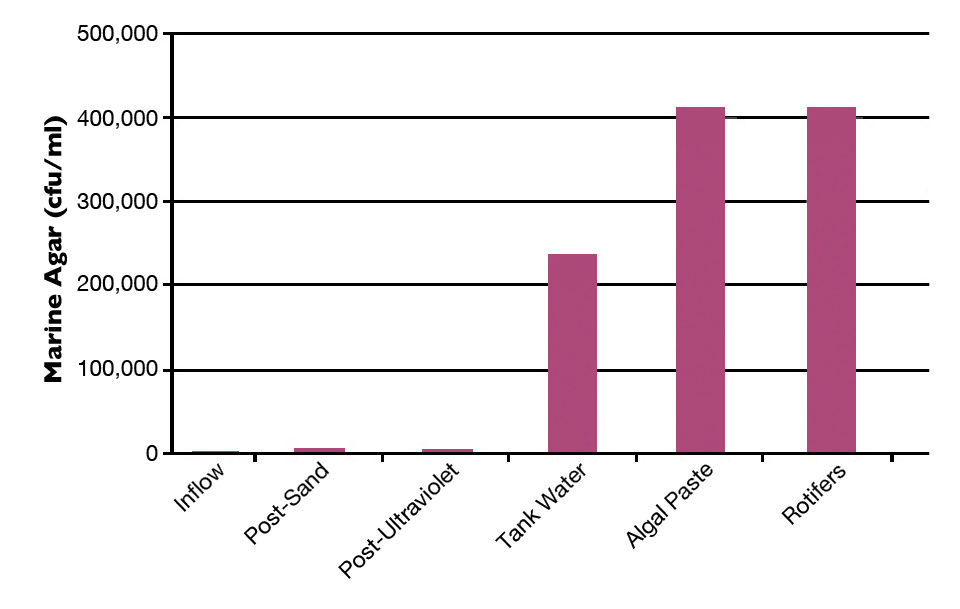
Although the incoming water for hatchery operations tended to have relatively low levels of marine cultivable bacteria relative to other examined vectors (Fig. 1), data suggested the waterborne bacterial complement had significant impacts on hatchery operations. Initial screens showed that bacteria titers in the water supply had remarkable fluctuations from less than 100 to over 6,000 cfu/ml, despite using water from a rather constant saltwater well system.
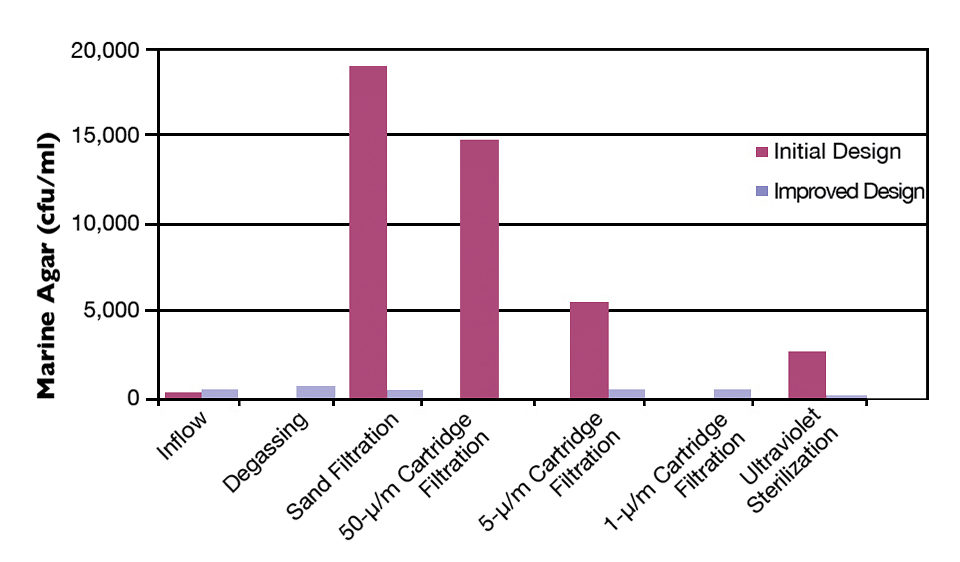
Hatchery run “crashes” were associated with periods of higher levels of cultivable bacteria. Examination of the incoming water source revealed that despite their critical role in protecting the hatchery, the water treatment and delivery systems provided many sites for bacterial proliferation and often added to the bacterial loads that reached the larval-rearing systems. Problem sites included pressurized sand filters and cartridge filters, along with piping, valves and equipment by-passes. Further, sand filter chlorination appeared to make the issues worse.
Design changes
Therefore, both the flow-through and recirculating water treatment systems used in the hatchery operations were redesigned. The design changes included a switch from sand to crushed glass as the initial solids filter to facilitate media cleaning, additional cartridge filtration, optimization of pipe sizes to facilitate self-scouring, and reduction of loops, by-passes, valves and other regions where bacteria might accumulate.
Water-sampling ports were incorporated after each treatment process to facilitate system study. In addition, the systems were designed so that cleaning and sterilizing solutions could be easily circulated through all parts of the systems for routine cleaning between hatchery runs.
The authors found increased bacteria levels at system startup and following system sterilization, while many system components initially increased total system bacteria levels. Over a week to 10 days of operation, the bacterial levels stabilized, leading to an overall reduction in cultivable bacteria of over 200-fold from initial conditions (Fig. 2).
Perspectives
Although many findings appeared common-sense, there is little information available on microbial ecology in aquatic hatchery operations. The adoption of relatively simple microbial culture screening protocols led to a better understanding of what goes on at the microbial level during hatchery operations and subsequent changes in operational protocols and system designs. In addition, the adoption of recirculating technologies for hatchery operations appears to provide a more stable microbial background less prone to the rapid system fluxes seen under flow-through modes of operation.
Another approach to reduce pathogenic species is the introduction of probiotic bacterial strains into the larval-rearing system to facilitate system population by beneficial bacteria. Clearly, a better understanding of the hidden microbial world of hatchery ecosystems would be a welcome addition for hatchery managers. In this case, a combination of operational and system changes helped reduce the risks of pathogen entry to the larval-rearing systems and helped restore large-scale fingerling production.
(Editor’s Note: This article was originally published in the January/February 2010 print edition of the Global Aquaculture Advocate.)
Authors
-
Charles W. Laidley, Ph.D.
Oceanic Institute
Finfish Department
41-202 Kalanianaole Highway
Waimanalo, Hawaii USA 96795 -
Kimberly Pinkerton, M.S.
Oceanic Institute
Finfish Department
41-202 Kalanianaole Highway
Waimanalo, Hawaii USA 96795 -
Chatham K. Callan, Ph.D.
Oceanic Institute
Finfish Department
41-202 Kalanianaole Highway
Waimanalo, Hawaii USA 96795
Tagged With
Related Posts

Health & Welfare
A comprehensive look at the Proficiency Test for farmed shrimp
The University of Arizona Aquaculture Pathology Laboratory has carried out the Proficiency Test (PT) since 2005, with 300-plus diagnostic laboratories participating while improving their capabilities in the diagnosis of several shrimp pathogens.

Health & Welfare
A holistic management approach to EMS
Early Mortality Syndrome has devastated farmed shrimp in Asia and Latin America. With better understanding of the pathogen and the development and improvement of novel strategies, shrimp farmers are now able to better manage the disease.

Responsibility
A look at various intensive shrimp farming systems in Asia
The impact of diseases led some Asian shrimp farming countries to develop biofloc and recirculation aquaculture system (RAS) production technologies. Treating incoming water for culture operations and wastewater treatment are biosecurity measures for disease prevention and control.

Health & Welfare
AHPN inferences based on behavior of vibrio bacteria
Vibrio parahaemolyticus, a strain of which is the cause of acute hepatopancreatic necrosis (AHPN), has both virulent and benign strains. This strain colonizes the stomachs of shrimp by the formation of a biofilm, which protects it from antibiotics and other potential treatments.


