Breeding program for SPF stocks

The black tiger shrimp, Penaeus monodon, was once the most prominent internationally traded shrimp species due to its fast growth, large sizes and widespread distribution throughout the IndoPacific region. Over time, the reliance of this sector on wild-caught spawners led to an increasing incidence of diseases that caused reduced survival, growth, feed efficiency and reproductive performance. Ultimately, health issues and associated declines in economic viability of infected wild P. monodon caused a dramatic shift to specific pathogen-free (SPF) genetically improved Pacific white shrimp, Litopenaeus vannamei.
To regain the outstanding growth potential of P. monodon will require SPF genetically improved stocks. As described in the previous article in this series, the Aquatic Animal Health and Service Centre was developed in Brunei to conduct molecular and histological tests for detecting the full range of shrimp diseases. Such a comprehensive diagnostic lab is a prerequisite for developing and maintaining SPF populations.
Development of SPF stocks
Wild adults collected from the coastal waters of Brunei were induced to spawn in a primary quarantine facility. If no diseases were detected in the parents, the nauplii of each spawn were recorded as a potential founder family and transferred to a secondary quarantine facility in a different location.
These F1 offspring were raised through the larval, juvenile, subadult and adult stages with repeated diagnostic testing. If no diseases were detected, they were induced to mature and spawn. If no diseases were detected in the spent parents, the resulting F2 offspring were considered SPF and introduced to the genetic nucleus at the Aquaculture Development Centre (ADC).
The ADC is a pre-existing facility that was modified to serve as an SPF P. monodon breeding center. It is equipped to rear all life stages in a highly biosecure environment.
Water treatment
Raw seawater is a potential disease vector. Consequently, the ADC seawater treatment and storage system is oversized with redundant treatment steps to assure a continuous supply of high-quality, disinfected water. Seawater is drawn from the ocean through a 300-m pipeline to a sump above the beach, where the water is pumped through pressurized sand filters and ozone disinfection to stacked reservoirs with a combined capacity of 1,200 m3. Settled water is pumped through additional filters and ultraviolet sterilizers to the culture systems. Discharged water is collected in a concrete sump for sedimentation and batch chlorination before release.
To further reduce the risk of water-borne disease, culture systems are designed to minimize water exchange through the use of biofloc systems for growout and use of recirculation systems with nitrifying biofilters for conditioning and maturation.

Biosecurity procedures
All culture tanks and raceways are located indoors and have bird netting covering any open sides. Access to the facility is controlled by 24-hour security. Authorized personnel enter through a single passage with a station for a mandatory change of shoes to boots. Footbath and hand wash stations are located at the entrance of each internal culture area.
Natural feeds are sourced from regions with low disease risk. Despite the fact that only SPF families enter the facility, every tank is considered potentially infected. Therefore, hands and equipment that touch the water in any tank must be disinfected before use in another tank. In addition, comprehensive disease surveillance is maintained throughout the life cycle of each family to assure continued SPF status.

Breeding procedures
The nucleus-breeding program began in 2008. It consists of fiberglass tanks for maturation, spawning, hatching, larviculture and nursery. Raceways of 25-m2 volume are used for juvenile rearing to adult size, and 50-m2 circular concrete tanks are used for reproductive conditioning. Supporting systems are in place for algae culture, Artemia, water quality testing, feed storage and preparation, equipment maintenance and backup electrical power.
Breeding is based on a family-selection process with each spawn of a unique male and female combination considered a family. The facility was originally designed to produce 30 families per breeding cycle with four cycles yearly. However, the maturation facility is currently being expanded from 70 to 240 m2 of net tank area to enable increased production of families and greater intensity of selection. In addition, a production-scale larviculture unit is being added to facilitate larger-scale pond trials.
Each family is reared separately through spawning, hatching, larval rearing and nursery stages until the animals reach sizes of 2 to 3 g. At that point, individuals within each family are tagged with a colored fluorescent elastomer injected in a specific segment of their abdominal muscles to enable family identification. Once tagged, individuals from all families within a batch are reared communally to eliminate family differences in their culture environment.
The breeding program has produced the fifth generation. To minimize loss of valuable genes during the first years of domestication, initial selection decisions have been designed to select crosses that avoid inbreeding and improve overall performance in reproduction, larval rearing and growout.
Both indoor raceways and biosecure ponds are used to rear tagged juveniles to selection size and then onward to adult size. Approximately 30 tagged juveniles from each family are reared in the raceways, while 250-300 juveniles/family are reared in ponds. The pond facility consists of four, 3,000-m3 units with concrete side walls, high-density polyethylene-lined bottoms, center drains, bird netting and crab fencing. All water is filtered, held in a sedimentation pond and chlorinated before use.
The growout ponds are stocked at low density to maximize growth rate and produce broodstock-size animals in a minimum time period. The animals are transferred on a regular basis, and fast-growing individuals are identified by family and tagged for later use in the selection program.
Juveniles reared in these biosecure ponds grow approximately 5 g/week and reach 100 g by 10 months of age (Figure 1). In contrast, juveniles reared at higher density in indoor raceways grow at only half this rate. However, they serve as a valuable biosecure backup population in the event of disease contamination in the ponds.
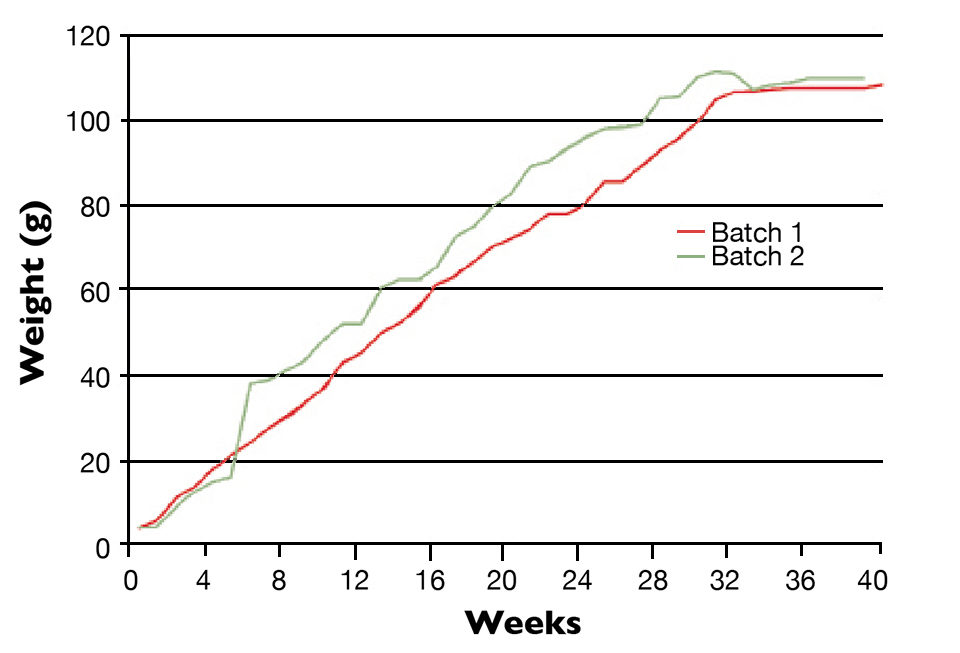
A sample of the pond animals is sent to the Brunei Department of Fisheries’ Aquatic Animal Health Services Centre for analysis by polymerase chain reaction and histology. If no diseases are detected, the pond is harvested, and selected individuals are returned to the breeding center for at least one month of reproductive conditioning. Both natural and artificial mating are used.
(Editor’s Note: This article was originally published in the September/October 2012 print edition of the Global Aquaculture Advocate.)
Authors
-
Chris Howell
Integrated Aquaculture International
3303 West 12th Street
Hastings, Nebraska 68902-0609 USA -
Hjh Rosinah Hj Yussof
Department of Fisheries
Brunei Darussalam -
Emma Farhana A Pakar
Department of Fisheries
Brunei Darussalam -
Siti Nurjuriah Hj Tengah
Department of Fisheries
Brunei Darussalam -
Abidah Hj Mohd Yazid
Department of Fisheries
Brunei Darussalam
Tagged With
Related Posts

Health & Welfare
Brunei project develops technology for large black tiger shrimp production, part 5
The final article in a series about a project in Brunei to develop technology for production of black tiger shrimp focuses on the development of advanced grow-out ponds and practices with enhanced systems for biosecurity, sludge removal, water reuse, energy efficiency, automatic feeding and mechanical harvesting.

Health & Welfare
Brunei project develops technology for large black tiger shrimp production, part 4
Nutrition research run in conjunction with the breeding program ensures that cost-effective feeds are optimized for shrimp performance for a five-year project undertaken in Brunei Darussalam to develop advanced technology for production of large black tiger shrimp.

Health & Welfare
Brunei project develops technology for large black tiger shrimp production, part 2
For a five-year project undertaken in Brunei Darussalam to develop advanced technology for production of large black tiger shrimp, a comprehensive disease diagnostic laboratory was developed to enable detection of known and emerging shrimp pathogens by molecular and histological methods.

Health & Welfare
Brunei project develops technology for large black tiger shrimp production, part 3
Specific pathogen-free offspring developed from founder populations of P. monodon collected from Brunei coastal waters were introduced into a biosecure breeding program, where they have been reared to the fifth generation using family-based selection for reproduction and growth.


