Conservation Fund’s Freshwater Institute evaluates alum and ferric chloride

Phosphorus is one of the most scrutinized nutrients discharged by aquaculture systems, due to its potential eutrifying impact on receiving bodies of water. Phosphorus is often the limiting nutrient in natural ecosystems, and excessive algae blooms can occur if discharges exceed the ecosystems’ absorption capacities.
The potential impacts of phosphorus on the environment and discharge limitations imposed by regulatory agencies have stimulated research on ways to reduce phosphorus in discharges from recirculating aquaculture systems. Because they provide a concentrated waste stream, recirculating systems provide opportunities for more efficient and economical phosphorus control. In contrast, for systems such as raceways, equivalent treatment of the dilute effluent flow stream would be difficult from both from engineering and economic standpoints.
Institute studies
Over the last decade, the Conservation Fund’s Freshwater Institute in Shepherdstown, West Virginia, USA, has demonstrated ways to reduce the wastes generated during aquaculture production, including improved feed and feeding strategies, and technologies to minimize water use and concentrate waste streams. A recent project evaluated alum and ferric chloride, two commonly used coagulation/flocculation aids used to treat the effluent from microscreen filters in intensive recirculating aquaculture systems.
In addition to determining the effectiveness of the aids in removing both suspended solids and phosphorus, systematic testing of the variables normally encountered in the coagulation/flocculation process was conducted. Tests evaluated the dosages and conditions required to achieve optimum waste capture.
Coagulation/flocculation aids
Electric charges on small particles in water cause the particles to naturally repel each other and keep them in suspension. One of the most commonly used methods for the removal of suspended solids from drinking water is the addition of coagulant and flocculation aids such as alum, ferric chloride, and long-chain polymers.
The coagulation process neutralizes or reduces the negative charge on particles. This allows the van der Waals forces of attraction between molecules to encourage the initial aggregation of colloidal and fine suspended materials to form microflocs. Flocculation is the process of bringing together the microfloc particles to form larger agglomerations by physically mixing the flocculants or through the binding action of flocculants such as long-chain polymers.
Through this process, it is possible to treat incoming or effluent aquaculture water to very high “drinking water” quality, thus allowing the placing of recirculating aquaculture systems on either impaired water supply resources or exceptionally high-quality receiving water.
Optimizing use
Since coagulant interactions are very complex, laboratory studies are used to determine the optimal dosage, duration, and intensity of mixing and flocculation. For over 50 years, the jar test has been the standard technique used to optimize the addition of coagulants and flocculants in the wastewater and drinking-water treatment industries.
During this study, samples for jar tests were taken directly from the effluent backwash water of several rotating microscreen filters used for suspended solids removal in two commercial-size recirculating production systems growing arctic charr.
Three steps
The coagulation/flocculation process consisted of three distinct steps. First, the coagulant was added to the effluent water and rapid, high intensity mixing was initiated. The objective was to obtain complete mixing of the coagulant with the wastewater to maximize the destabilization of colloidal particles and initiate coagulation. The critical parameters for this step were duration and paddle speed, or mixing intensity, referred to as the velocity gradient.
Second, the suspension was slowly stirred to increase contact between coagulating particles and facilitate the development of large flocs. Again, the flocculation duration and intensity were critical parameters. Too high an intensity could break up the aggregate flocs, and too low could allow them to settle out too soon.
Third, mixing was terminated and the floc was allowed to settle.
Test setup
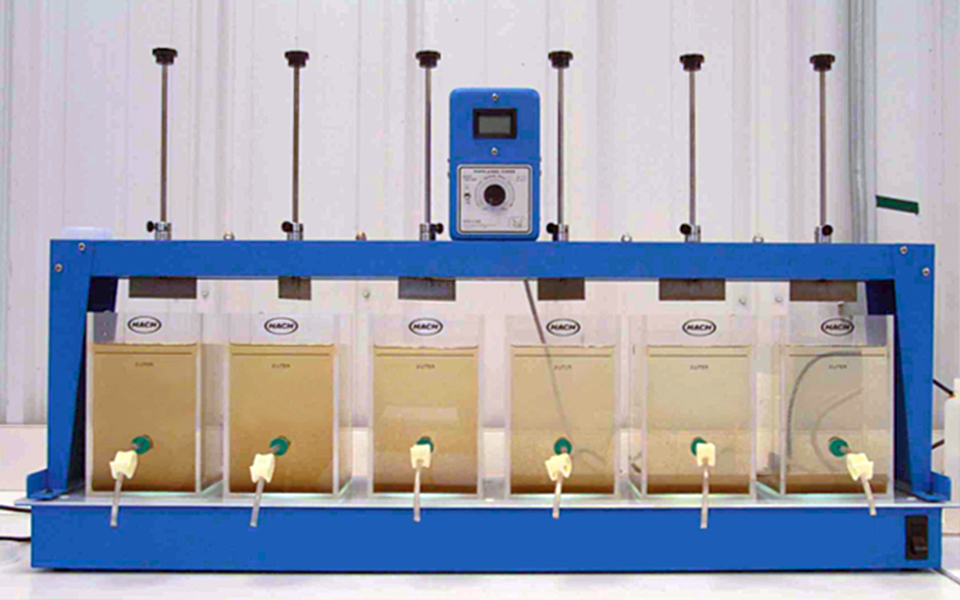
A standard jar test apparatus with six square 2-liter plexiglass jars was employed for the tests. The six flat stirring paddles were all driven by a single variable-speed motor from 0 to 300 rpm.
For all the jar tests, pH, turbidity, and soluble reactive phosphorus (SRP, or orthophosphate) were measured. For comparing the effects of operating parameters such as mixing and flocculation speed, turbidity was used as an indicator of suspended solids, with orthophosphate used for phosphorus content.
Results
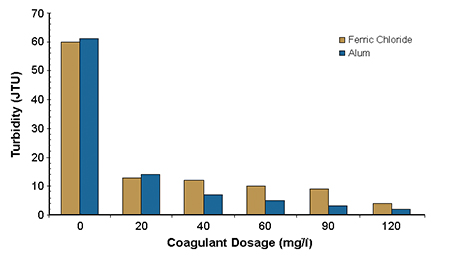
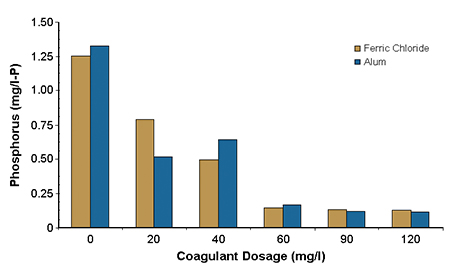
Fig. 1 shows a comparison of the effectiveness of alum and ferric chloride in removing orthophosphate. Fig. 2 shows turbidity from the microscreen backwash water.
At concentrations above 60 milligrams per liter, orthophosphate concentrations were reduced to about 0.15 milligrams per liter-phosphorus for both alum and ferric chloride. At 60 milligrams per liter, the turbidity approached its lowest value. Mixing intensity had only a minor impact on removal efficiency, as did flocculation time and intensity. Settling time requirements were less than 10 minutes to achieve maximum removal of both orthophosphorus and suspended solids.
Conclusion
Testing results indicated that both alum and ferric chloride were effective in the removal of soluble orthophosphates and suspended solids from water. Further work at the Freshwater Institute is assessing several commercially available polymer flocculation aids and examining how best to utilize coagulant/flocculation aids as pretreatment before traditional solids settling basins or in more comprehensive solids management systems.
(Editor’s Note: This article was originally published in the December 2003 print edition of the Global Aquaculture Advocate.)
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Authors
-

J.M. Ebeling, Ph.D.
The Conservation Fund’s Freshwater Institute
1098 Turner Road
Shepherdstown, West Virginia 25443 USA -
Sarah R. Ogden
The Conservation Fund’s Freshwater Institute
1098 Turner Road
Shepherdstown, West Virginia 25443 USA -
Kata Rishel
The Conservation Fund’s Freshwater Institute
1098 Turner Road
Shepherdstown, West Virginia 25443 USA
Tagged With
Related Posts

Aquafeeds
A look at corn distillers dried grains with solubles
Corn distillers dried grains with solubles are an economical source of energy, protein and digestible phosphorus to reduce feed costs and fishmeal usage.

Responsibility
Chemical fertilizers in pond aquaculture
Chemical fertilizers are frequently used in pond aquaculture to stimulate phytoplankton productivity and enhance the availability of natural food organisms.

Health & Welfare
Dissolved oxygen dynamics
Dissolved oxygen management is the most important requirement of aquaculture pond water quality. DO concentration below 3 mg/L is stressful to shrimp.

Responsibility
Inorganic fertilization of production ponds
Nitrogen and phosphorus are the most important nutrients in fertilization of both freshwater and coastal ponds. Many factors that can affect the response of ponds to fertilizers are location-specific. Aquaculture pond managers will have to figure out the best procedure for a given location or even for an individual pond.


