Use a combined diet until completion of larval period

Early postmetamorphic stages of most freshwater and marine fish feed mainly, if not exclusively, on zooplankton. As fish larvae develop, their dietary requirements change and individuals begin to adopt adult dietary habits.
The importance of a complete diet has been a long-standing debate in the rearing of tilapia. Previous studies that examined the early dietary requirements of tilapia routinely used individuals that were several days to weeks past first feed, which occurs approximately seven to eight days after fertilization in Nile tilapia, (O. niloticus). These studies assumed that tilapia were morphologically and physiologically competent to consume formulated feeds at first feed.
Other investigators assumed that tilapia larvae feed primarily on phytoplankton or unattached bacteria. The bacteria were then acid hydrolyzed and enzymatically digested within the stomach.
These assumptions may not be correct. Studies with tilapia at the University of Alabama in Birmingham, Alabama, USA, have shown that formulated diets, algae, and bacteria can be digested only after the alimentary canal has achieved a specific morphological and physiological competency. This occurs at the end of the larval period several days after feeding has been initiated.
Live prey necessary?
An evaluation of the buccal/pharyngeal cavity of larval Nile tilapia at first feed shows an array of sharp papilliform teeth situated perpendicular to the central axis in a posterior-medial orientation, with a slightly curved appearance. These teeth appear to be adept at grasping and macerating live prey. Based on their spacing and organizational patterns, the teeth are adapted for larger prey such as zooplankton rather than smaller phytoplankton and bacteria.
Observations of these larval fish indicate they are sight feeders that quickly taste moving objects in the water column. Consequently, is live prey necessary for optimal weight gain in larval Nile tilapia?
Larvae study

In a project supported in part by the United States Department of Agriculture, the authors recently conducted a study at the University of Alabama to address this question. Approximately 900 embryos from each of three brooding Nile tilapia females were collected and incubated in McDonald jars in a recirculating system. The embryos entered the larval stage as free-swimming fry seven days after fertilizatio
At this time, all larval fry were removed from the jars, informally separated into three diet treatments, and placed in 80-l glass aquariums at approximately 300 fry per aquarium. Each treatment was replicated three times using fry from different broodstock.
The dietary treatments were HP, a size #00 commercial diet with 50 percent protein; A, stage I artemia franciscana nauplii; and A/HP, a combination of artemia nauplii and the high-protein diet. The hatched artemia nauplii were added to treatment tanks where applicable to achieve a final density of approximately 5 artemia per milligrams.
The diets were fed to the larvae at 8:00 a.m., 1:00 p.m., and 5:00 p.m. each day. Throughout the experimental period, levels of artemia nauplii were held constant and the high-protein diet was fed ad libitum. On the first and second day of feeding, larvae fed primarily in the water column. After the second day, larvae fed from both the water columns and bottoms of the tanks.
Twenty-five individuals were removed from each replicate of each treatment and weighed individually at three, seven, 10, 12, 14, 21, 22, and 27 days postfertilization (DPF). Each individual was blotted quickly to remove excess water, placed onto a tarred plastic weighing boat, weighed, and placed back into its respective tank. This method did not cause mortality in any treatment.
Mean wet weight measurements were compared using a one-way ANOVA. Comparison of the variances among replicates was first analyzed using Bartlett’s test. In all treatments, significance was determined using a probability value of P < 0.05.
Results
Feeding was initiated at 8 DPF. Significant differences in wet weight gain were not observed among treatments until 12 DPF, after which the fish fed A/HP grew 70 percent larger than the fish in the other treatments (Fig. 1). There was no significant difference in weight gain between A and HP treatment individuals throughout the study, although HP individuals, on average, were slightly larger.
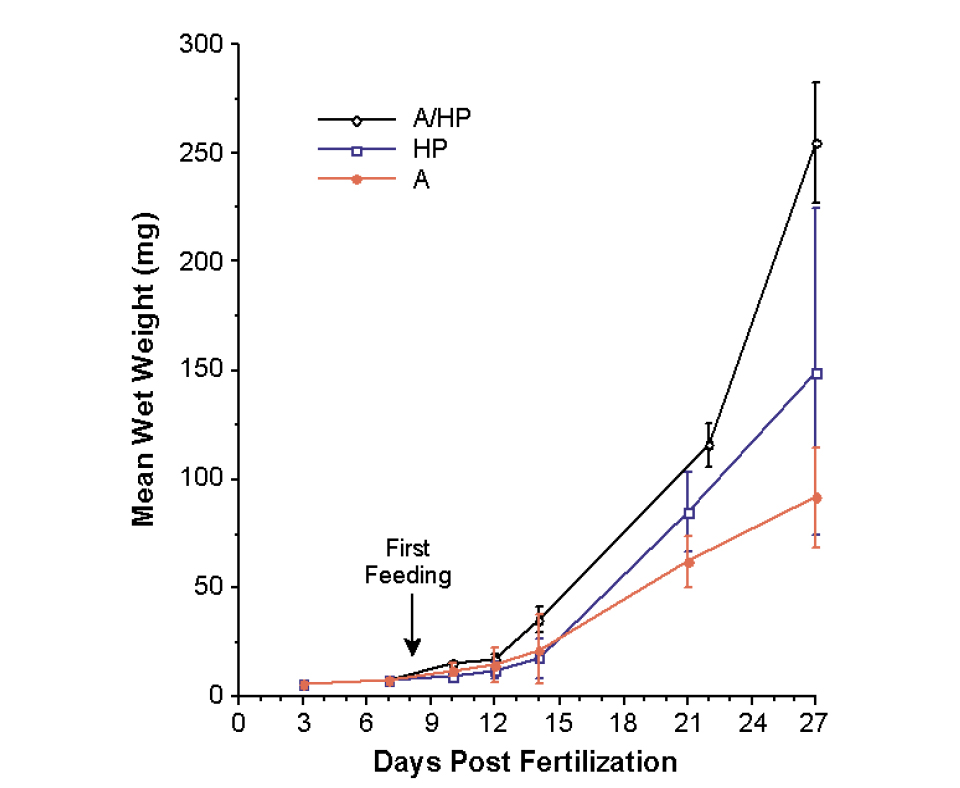
At day 27, the 11 percent coefficient of variation in fry fed the A/HP diet was much lower than that for fish fed the A or HP diets (25 and 50 percent, respectively). From 8 to 27 DPF, the mean weight of the A/HP individuals increased at a rate of 12.36 milligrams per day, compared to 7.07 milligrams per day for HP individuals and 4.7 milligrams per day for A individuals. The percentages of daily weight gain were 17.5, 14.7, and 12.2 in the A/HP, HP, and A treatments, respectively. Survivorship was greater than 98 percent in all treatments.
Conclusion
In a study, a combination of live artemia and a formulated diet high in protein during the first several days of feeding enhanced weight gain and reduced the coefficient of variation in larval Nile tilapia as compared to either component alone. The authors suggest the use of the combined diet until the completion of the larval period (approximately 12 days).
The mechanism by which live diets promote growth is not well understood. Visual stimulation by the movements of live prey has been suggested to enhance feeding by initiating instinctual behaviors of prey capture. Additionally, artemia nauplii may be effective at promoting growth simply because they are a source of readily digestible, high-energy nutrients. Artemia alone, however, may not contain adequate nutrients for postlarval growth, and the energy required for artemia or other zooplankton capture would be prohibitive in larger fry.
If tilapia fry do not receive an adequate diet during early larval development, the alimentary canal, accessory digestive organs, and feeding apparatus will either delay or not fully develop, and the fish may not realize their growth potential. Similar results have been observed in striped bass larvae.
(Editor’s Note: This article was originally published in the April 2004 print edition of the Global Aquaculture Advocate.)
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Authors
-
C. Daniel Bishop
Department of Biology
University of Alabama at Birmingham
Birmingham, Alabama 35294-1170 USA -
Stephen A. Watts, Ph.D.
Department of Biology
University of Alabama at Birmingham
Birmingham, Alabama 35294-1170 USA
Tagged With
Related Posts

Health & Welfare
Applied commercial breeding program for Nile tilapia in Egypt
A major goal of selective breeding program for Nile tilapia (Oreochromis niloticus) in Egypt is to select for fillet color and fillet weight in response to consumer preferences.

Health & Welfare
10 paths to low productivity and profitability with tilapia in sub-Saharan Africa
Tilapia culture in sub-Saharan Africa suffers from low productivity and profitability. A comprehensive management approach is needed to address the root causes.

Aquafeeds
A look at India’s fish feed industry
India's fish-farming industry makes limited use of modern feeds, providing potential for the feed sector to grow. Commercial feeds are predominantly used for pangasius farming, followed by a rising popularity in carp culture.

Health & Welfare
A look at aquaculture in Guyana
With its large quantities of water and little industry to pollute it, Guyana has the potential to become a greater player in global aquaculture.


