Improved growth, survival for postlarvae, juveniles grown under different conditions with Novacq

There has been an increased focus on water management strategies as a result of strict regulations on water and nutrient discharge in shrimp production globally. Due to the limits placed on water exchange and addition of different carbohydrates sources (high carbon:nitrogen, C:N ratio), alternative strategies have been adopted to maintain the water quality of these systems. For example, commercially available probiotics can be used to boost heterotrophic bacteria in the culture medium to control the ammonia peaks during early stages of production. This practice has increased in the past decade as a mean of controlling disease spread.
Alternatively, managing water exchange systems within intensive shrimp culture can facilitate the growth of photoautotrophic organisms (green water) that can keep both dissolved oxygen and pH stable. While the production of microorganisms in shrimp systems is a useful strategy to maintain water quality, the microorganisms also supply an external food source that can contribute to the positive effects observed on shrimp performance and health when cultured in these systems.
The nutritive benefits of microbial biomass have been characterized recently through feeding the commercially available, microbial biomass feed additive, Novacq™. Several papers (available from the corresponding author) have reported the benefits of this additive at 5 or 10 percent inclusion levels in diets for black tiger shrimp (Penaeus monodon) juveniles; these benefits include improvement in growth, survival, feed intake and nutrient retention efficiency. This additive has also shown positive outcomes when fed to P. monodon postlarvae in custard diets.
And concerning key ingredients in shrimp nutrition, Novacq™ was determined to be more bioactive than whole squid, krill meal and krill hydrolysate in terms of its performance and efficiency. The study suggests that shrimp fed a diet with a 10 percent inclusion of this commercial additive were able to use more efficiently dietary macronutrients for growth due to improvements in feed intake and post-absorptive processes. Most of these studies were carried out in clear-water tank systems, and only one of them in outdoor green-water tanks.
To date, there is no information whether this commercial additive supports similar results in culture conditions dominated by heterotrophic organisms, compared to the clear- and green-water conditions tested in prior studies. Here we present results of a study to provide initial insights on the effects of Novacq™ in different culture conditions (dominated by heterotrophic or photoautotrophic organisms), and the effect of nutritional history on performance in a clear water feeding trial.
The authors would like to thank Brian Murphy and Chris Stevenson for rearing the animals, and Sarah Frerichs, Isaak Kadel and Emmerson West for their assistance during the clear-water feeding trial. We also thank Barney Hines, Nicholas Bourne and Claudia Machado for their assistance with the proximate composition analyses.
Study setup
The control feed was formulated to contain fishmeal (500 grams per kg) and fish oil (15 grams per kg) as the primary protein and lipid sources, respectively. This basal formulation, previously validated, was supplemented with 100 grams per kg of marine microbial biomass (Novacq™ feed) at the expense of wheat flour and diatomaceous earth.
Ingredients were milled to 500 µm before batching, and the feeds were mixed with water and oil added to form a dough, which was blended to form fine crumbles. Those were spread on a mesh tray and steamed for 3 minutes and oven-dried at 65 degrees-C for 24 hours. Adequate pellet sizes were manufactured following the above procedures according to animal growth.
Rombenso, Novacq, Table 1
| INGREDIENTS | CONTROL DIET | NOVACQ DIET |
|---|---|---|
| Novacq | 0 | 10 |
| Fishmeal | 50 | 50 |
| Wheat gluten | 7 | 7 |
| Wheat flour | 34.03 | 28.03 |
| Fish oil | 1.5 | 1.5 |
| Soy lecithin | 1 | 1 |
| Stay-C | 0.1 | 0.1 |
| Vit premix (shrimp) | 0.2 | 0.2 |
| Mineral premix | 1 | 1 |
| Cholesterol | 0.1 | 0.1 |
| Diatomaceous earth | 5 | 1 |
| Astaxanthin | 0.05 | 0.05 |
| Banox E | 0.02 | 0.02 |
| PROXIMATE COMPOSITION % | – | – |
| Dry matter | 94.1 | 96.2 |
| Protein | 43.7 | 41.2 |
| Lipid | 9.1 | 8.1 |
| Ash | 14.2 | 14.4 |
| Gross energy (KJ/g) | 19.2 | 19.4 |
P. monodon postlarvae from four different families bred and reared at Bribie Island Research Centre, BIRC (Queensland, Australia) were combined and reared at the BIRC hatchery system. Shrimp were fed a ration of artemia and commercial post-larval feed until they were moved to cages held in raceways for Experiment 1.
For detailed information on statistical analyses used, contact the corresponding author.
Experiment 1: PL cage culture in raceways with heterotrophic or autotrophic water
Postlarvae (PL19 to 24; average weight of 5.12 ± 0.37 mg, grand mean ± SE) were randomly stocked in sixteen 512-L cages (80 x 80 x 80 cm) equally distributed in two 9 × 2.4 × 1 meter covered, outdoor raceways (eight cages per raceway) at a stocking density of 550 shrimp per cage. Shrimp were acclimated to the system on a commercial diet (shrimp feed from Ridley Corp.) for one week before being offered the diet treatments. The two diet treatments were randomly allocated to achieve four replicate cages per raceway.

Two culture conditions designated as CHO (a raceway with water rich with heterotrophic organisms and carbohydrate additions) and GREEN (a raceway with water rich with photoautotrophic organisms) were tested in this experiment, and characteristics and water quality parameters are summarized in Tables 2 and 3. The raceway with CHO culture conditions was filled 17 days prior to stocking with 60 percent clean treated water (treated with ultraviolet, UV, and ozone) and then the remaining 40 percent volume with 14-day old matured water from an adjacent “start-up” raceway.
Rombenso, Novacq, Table 2
| CONDITION 1 (CHO) | CONDITION 2 (GREEN) |
|---|---|
| Dominated by heterotrophic organisms | Dominated by photoautotrophic (phytoplankton) organisms |
| Limited water exchange (<2.5/% day) and inoculation of mature water (inoculum at a rate of 40% 17 days prior to stocking) | Water exchange (5-10% day) |
| Carbohydrate addition (molasses and C:N 11:1 – from Emerenciano et al 2017) | No Carbohydrate addition |
| Commercial water probiotics addition | No water probiotics addition |
Rombenso, Novacq, Table 3
| PARAMETER | CONDITION 1 (CHO) | CONDITION 2 (GREEN) |
|---|---|---|
| Dissolved oxygen (mg/L) | 5.18 ± 0.45 | 5.27± 0.49 |
| Temperature (degrees-C) | 26.60 ± 1.20 | 26.67 ± 2.14 |
| pH | 8.16 ± 0.23 | 8.30 ± 0.25 |
| Salinity (ppt) | 35.28 ± 1.40 | 35.30 ± 1.16 |
| TAN (mg/L) | 1.64 ± 2.37 | 2.01 ± 3.05 |
| NO2 (mg/L) | 4.96 ± 7.49 | 0.29 ± 1.01 |
| NO3 (mg/L) | 21.43 ± 33.13 | 5.71 ± 9.40 |
| Alkalinity (ml/L) | 179.31 ± 19.36 | 153.69 ± 26.86 |
| Settling solids (ml/L) | 1.29 ± 1.24 | 0 ± 0 |
Throughout this 31-day maturation phase (17+14 days) prior to stocking, the system received applications of a commercial water probiotic, molasses (carbon source), powdered zeolite (suspended artificial substrate), sodium bicarbonate (pH buffer) and a blend of commercial shrimp feed and urea (nitrogen sources) to stimulate the development of heterotrophic and nitrifying bacteria communities. Applications of “pre-activated” probiotics and molasses (one to two weekly applications) maintained a high C:N ratio throughout the trial phase until the conclusion of the experiment. Water was partially exchanged only when ammonia or nitrite exceeded 4 ppm, and when sludge accumulated underneath the trial cages.
The raceway with GREEN culture conditions was filled three days prior to stocking with 100 percent clean treated water (UV and ozone treated). This system received no additives before stocking or during the trial phase, other than minor pH buffering (additions of sodium bicarbonate). Water was exchanged when ammonia or nitrite exceeded 1 ppm or if suspended solids appeared in the water column (none observed during the trial phase).
Postlarvae were hand-fed twice daily (08:30 a.m. and 3:30 p.m.) seven days a week until apparent satiation, as determined by the presence of uneaten feed on trays. Feed to apparent satiation was adjusted daily ensuring under- or overfeeding did not occur. After 75 days of the feeding trial, all animals were individually weighed to assess growth and survival.
Fig. 1 shows the final biomass and survival of shrimp in this experiment with the two dietary treatments and the two different culture conditions. Although no statistical differences were observed, the final biomass and survival in the Novacq™ groups were 7 and 20 percent higher than for the control group, respectively, in the CHO raceway. Under GREEN conditions, animals fed the additive diet had a final biomass 31 percent larger than animals fed the control diet. And for shrimp fed the additive diet, those reared in GREEN conditions had 17 and 20 percent higher biomass and survival, respectively, compared shrimp those reared in CHO conditions.
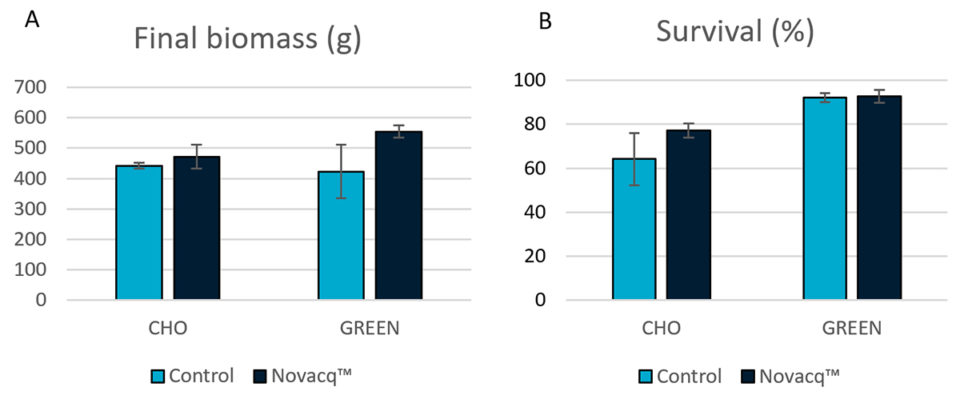
Experiment 2: Juvenile cage culture in raceway with heterotrophic (CHO) water conditions
In this 50-day experiment, we used juvenile shrimp (average weight 1.56 ± 0.06 grams, mean ± SE) from Experiment 1 already exposed to the control and the additive diets during their growth from PL to juveniles. These juveniles were stocked in eight 512-liter cages (80 x 80 x 80 cm), equally distributed and randomly placed within one outdoor, covered raceway (9 x 2.4 x 1 meters) with CHO water and at a stocking density of 100 shrimp per cage. Culture condition, water quality monitoring, feeding and dietary treatments were the same as previously described for Experiment 1.

Rombenso, Novacq, Table 4
| PARAMETER | CONDITION 1 (CHO) |
|---|---|
| Dissolved oxygen (mg/L) | 4.91 ± 0.46 |
| Temperature (degrees-C) | 26.28 ± 1.32 |
| pH | 7.86 ± 0.11 |
| Salinity (ppt) | 31.02 ± 2.66 |
| TAN (mg/L) | 0.90 ± 0.2 |
| NO2 (mg/L) | 3.70 ± 4.4 |
| NO3 (mg/L) | 73.0 ± 60.0 |
| Alkalinity (ml/L) | 179.31 ± 19.36 |
| Settling solids (ml/L) | 1.29 ± 1.24 |
In Experiment 2 we observed similar trends to those in Experiment 1, but animals fed the additive diet statistically outperformed shrimp fed the control diet. The final biomass and survival in shrimp fed the additive diet were 16.9 and 40.8 percent higher than for the control diet group. We observed a high variation in survival within the control feed group compared to the additive diet group, and that continually using Novacq™ feeds for a total of 125 days (75 days in Experiment 1, 50 days in Experiment 2) under CHO culture conditions appeared to improve shrimp performance compared to animals in the control treatment.
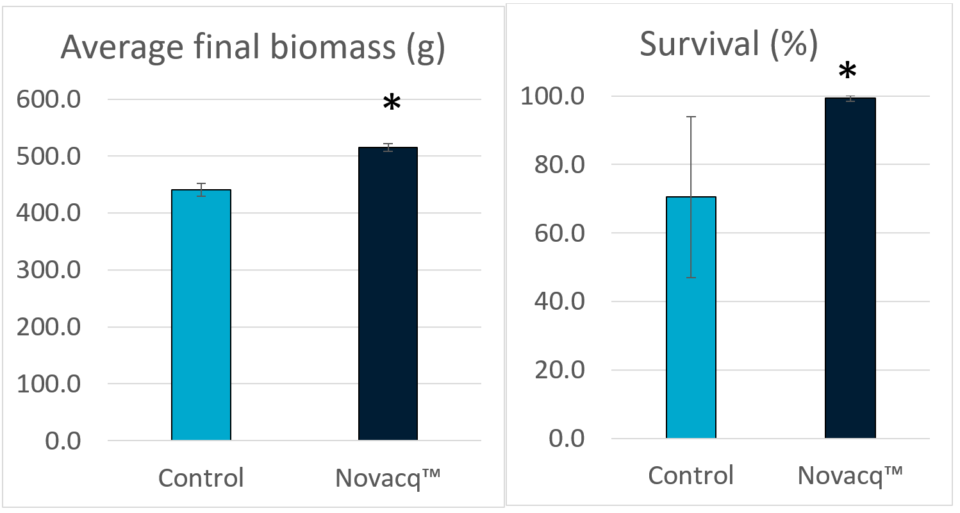
Experiment 3: Juvenile culture in indoor, clear water tanks
After completion of Experiment 1, some juvenile shrimp (average weight 1.00 ± 0.02 grams; grand mean ± SE) from both rearing conditions were transferred to an indoor facility, and randomly distributed into sixteen, 80-liter tanks at 15 shrimp per tank. This indoor system had flow-through, heated, 30 ppt seawater at 0.7-liters per minute per tank. During this experiment, water temperature and dissolved oxygen (DO) were monitored daily and averaged 29.8 ± 0.2 degrees-C and above 5.0 mg per liter.

Shrimp were allocated the same dietary treatments they had in Experiment 1 – animals that were fed the control or additive diet received the same diets in Experiment 3. Additionally, some shrimp from the CHO raceway in Experiment 1 were distributed in a factorial dietary design where animals fed the control diet in Experiment 1 were now offered the additive diet, and vice-versa. Due to logistics limitation, this factorial design was not expanded to the GREEN raceway.
Feeds used in this experiment were manufactured following the CSIRO-standard procedures as described earlier. Shrimps were fed to apparent satiation five times a day, once manually (11 a.m.) and four times using automatic feeders (6 p.m., 9 p.m., midnight, 3 a.m.). Tank bottoms were siphoned daily.
The dietary trends observed in Experiments 1 and 2 were accentuated when shrimp were assessed in the indoor, clear water tank system. At the end of Experiment 3, animals initially reared under CHO conditions in Experiment 1 and fed the NovacqTM diet had a weight gain 210 percent larger compared to shrimp that received the control diet (P < 0.05). Similarly, shrimp initially reared under GREEN conditions in Experiment 1 and fed the additive diet had a weight gain 109 percent larger compared to animals fed the control diet. We believe the noticeable effect of the additive diet could have been a consequence of prior exposure of the animals to this diet (nutritional history, and that further studies are warranted to evaluate the effects of NovacqTM supplementation in the context of nutritional history, especially in long-term studies (>2 months).
Results and discussion
Overall, our results show that P. monodon PLs and juveniles fed diets with the commercial additive NovacqTM for extended periods of time (Experiments 1, 2 and 3) produced higher growth in the experimental, as illustrated by average individual final weights in Fig. 3, when compared to animals fed the control diet, regardless of the culture conditions. Additionally, feeding the additive diet to shrimp previously fed the control diet improved their growth, while the reverse dietary treatment impaired animal growth (Fig. 3).
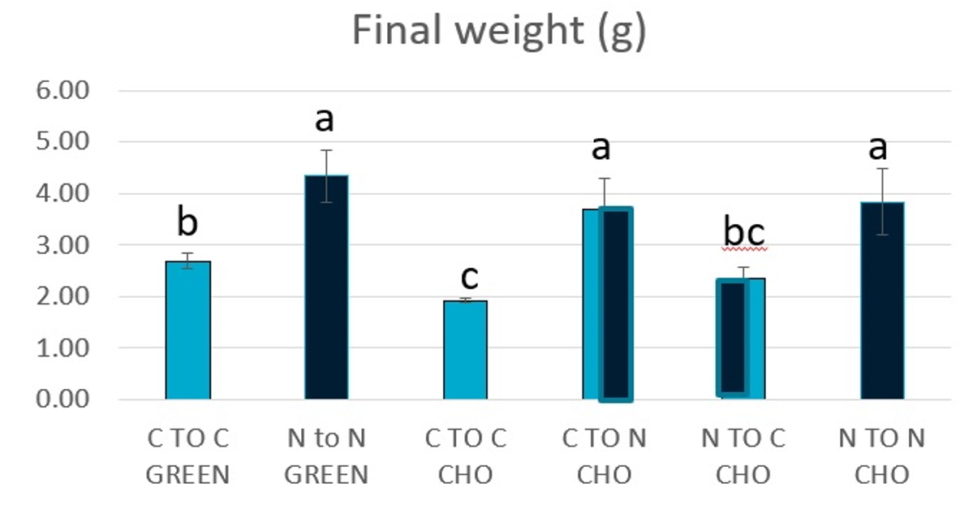
Survival was not significantly different (P > 0.05) between diets or culture conditions. However, shrimps reared under GREEN conditions had an average 6 percent higher survival than shrimp grown under CHO conditions. We believe the benefits in growth and condition of shrimp reared in GREEN, as observed in Experiment 1, were carried on to the clear water system, and were likely linked to better nutritional conditions. Although the shrimp were initially stocked at a similar weight, animals reared under GREEN conditions had, on average, higher weight gain and survival than those reared under CHO conditions. This better performance is likely due to the amount and quality of the available nutrients in the GREEN as opposed to the CHO raceways.
These findings agree with various previous publications on the use of diets including NovacqTM in clear-water conditions. Similarly, under green water conditions, supplementation of this additive at 100 grams per kg in feeds containing different levels of fishmeal and oil was demonstrated to improve growth and feed intake compared to the respective control feed by some researchers. It is worthwhile noting here that our findings were achieved with the additive diet having 2.5 percent less protein content compared to the control feed, and therefore not due to more favorable protein to energy ratios (Table 1). Similarly, additive supplementation at 100 grams per kg successfully offset reductions in the dietary protein content varying from 36 percent (low protein treatment) to 54 percent (high protein treatment) without impairing final weight of juvenile P. monodon, as previously reported by other researchers.
It is well established that culture conditions affect shrimp performance and survival. In Experiments 1 and 2, we did not determine any statistical differences at first, but after 135 days the shrimp fed the additive diets had statistically higher final biomasses and survivals under the CHO water conditions when compared to the control diet. As with any feed additive, the degree of performance and survival improvements in shrimp fed NovacqTM will depend on the culture condition.
Perspectives
Previous studies by various researchers have reported the benefits of the commercial feed additive NovacqTM to shrimp – including improved survival, growth, feed intake and nutrient retention efficiency and utilization of bioavailable nutrients – compared to a standard reference feed.
Results of our study show that supplementation with this additive of diets for P. monodon PLs and juveniles produced improved shrimp growth and survival under the three culture conditions tested, including rich in autotrophic organisms (GREEN), rich in heterotrophic organisms (CHO) and clear water. We believe our findings support the positive impact of continually feeding feeds based on this commercial additive throughout the culture cycle to maximize production performance.
References available from first author.
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Authors
-
Artur Rombenso, Ph.D.
Corresponding author
Research Scientist – Animal Nutrition
CSIRO Livestock & Aquaculture Program
Bribie Island Research Centre, 144 North St, Woorim, QLD 4507 AUS[117,97,46,111,114,105,115,99,64,111,115,110,101,98,109,111,114,46,114,117,116,114,97]
-
Simon Irvin
Research project – Aquatic Stock Management
CSIRO Livestock & Aquaculture Program
Bribie Island Research Centre, 144 North St, Woorim, QLD 4507 AUS[117,97,46,111,114,105,115,99,64,110,105,118,114,105,46,110,111,109,105,115]
-
Ha Truong, Ph.D.
Post-doctoral fellow – Animal Nutrition
CSIRO Livestock & Aquaculture Program
Bribie Island Research Centre, 144 North St, Woorim, QLD 4507 AUS[117,97,46,111,114,105,115,99,64,103,110,111,117,114,116,46,97,104]
-
Dean Musson
Research technician – Aquatic Stock Management
CSIRO Livestock & Aquaculture Program
Bribie Island Research Centre, 144 North St, Woorim, QLD 4507 AUS[117,97,46,111,114,105,115,99,64,110,111,115,115,117,109,46,110,97,101,100]
-
Tim Perrin
Research technician – Aquatic Stock Management
CSIRO Livestock & Aquaculture Program
Bribie Island Research Centre, 144 North St, Woorim, QLD 4507 AUS[117,97,46,111,114,105,115,99,64,110,105,114,114,101,112,46,109,105,116]
-
David Blyth
Feed technologist – Animal Nutrition
CSIRO Livestock & Aquaculture Program
Bribie Island Research Centre, 144 North St, Woorim, QLD 4507 AUS[117,97,46,111,114,105,115,99,64,104,116,121,108,98,46,100,105,118,97,100]
-
Mauricio Emerenciano, Ph.D.
Research Scientist – Production Systems
CSIRO Livestock & Aquaculture Program
Bribie Island Research Centre, 144 North St, Woorim, QLD 4507 AUS[117,97,46,111,114,105,115,99,64,111,110,97,105,99,110,101,114,101,109,101,46,111,105,99,105,114,117,97,109]
-
Matthew Briggs, Ph.D.
Technical Project Manager
Ridley Aquafeeds
Unit 4 – 31 Robart Court, Narangba QLD 4504, AUS[117,97,46,109,111,99,46,121,101,108,100,105,114,64,115,103,103,105,114,98,46,119,101,104,116,116,97,109]
-
Cedric Simon, Ph.D.
Research Group Leader, Nutrition & Production Systems
CSIRO Aquaculture Program
Queensland Bioscience Precinct, 306 Carmody Road, St. Lucia, QLD 4067 AUS[117,97,46,111,114,105,115,99,64,110,111,109,105,115,46,99,105,114,100,101,99]
Tagged With
Related Posts

Aquafeeds
Aquafeed moonshots at the F3 ‘talent show’
At the F3 (fish-free feed) Companies Got Talent event in Burlingame, Calif., last week, alternative (non-marine) aquafeed ingredient companies spoke of decoupling aquaculture from fishmeal and fish oil in their quest for greater sustainability.

Aquafeeds
Functional ingredients driving shrimp feed innovation
Functional ingredients and additives promote growth, improve health of farmed shrimp, and bolster immune response and other physiological needs.

Health & Welfare
Efficacy of saturated lipids in juvenile California yellowtail feeds
This study compared production performance and tissue fatty acid composition of California Yellowtail fed diets containing various saturated lipids.

Aquafeeds
Evaluating dietary fish oil replacement in juvenile Florida pompano
Study evaluated production performance and tissue composition of juvenile Florida pompano fed diets containing fish oil or 25:75 blends of fish oil and various other lipid sources.


