Producers must earn public confidence that food safety and environmental risks are addressed

Commercialization of the first food products from transgenic fish is widely expected within the next few years. A leading aquaculture biotechnology company has already petitioned the United States Food and Drug Administration for approval to market genetically modified salmon, which sparked debate. Although still controversial, the potential benefits of transgenic fish and shellfish biotechnology can yet be realized, while minimizing risks to human health and the environment.
Potential benefits of gene transfer technology
A gene from any species can be introduced into a new species by injecting it, within a well-designed gene construct, into newly fertilized eggs of the intended host. Gene transfer poses several benefits for aquaculture. Most gene transfer experiments have introduced additional copies of a growth hormone gene into an aquaculture species in hopes of producing a faster-growing aquaculture stock.
A/F Protein, the company that aims to commercialize the transgenic Atlantic salmon, reports a four- to sixfold acceleration of growth rate, with a 20 percent improvement in the rate of conversion of feed to fish flesh. Experiments have also attempted to increase cold tolerance by introduction of “antifreeze” protein genes from Arctic or Antarctic fishes.
Improvement of other traits will depend on the identification of key genes – those conferring resistance to diseases or restoring the function of a biochemical pathway, such as those for synthesis of vitamin C or omega-3 fatty acids, for example.
Food safety
The prospect of marketing products from transgenic fish and shellfish raises the issue of their food safety. Key issues regarding the modified protein in the tissue of the animals are any potential biological activity, allergenicity, and toxicity.
Biological activity
Most proteins are digested prior to absorption and are pharmacologically inactive after oral consumption. In the case of an introduced growth hormone gene, non-primate growth hormone molecules are not biologically active in humans, nor are fragments of molecules, or other hormones secreted in response to growth hormone administration. If the new protein originates from a known allergenic source, or its amino acid sequence resembles a known allergen, the protein can be tested for reactivity with serum from individuals with known food allergies.
Allergenicity
A more difficult issue arises when a new protein comes from a source that is not historically a human food. However, evidence suggests little concern about proteins that are from sources with no history of allergenicity, that lack amino acid sequence similarities to known food allergens, are rapidly digested, and expressed at lower levels than major allergens.
Toxins
Toxins are well studied and would not be purposefully transferred into a food animal. Testing for toxicity is relatively straightforward, and would be required before a product is approved for marketing. Hence, food scientists have concluded that gene transfer is unlikely to result in foods that are less safe than their conventional counterparts.
Environmental safety
Commercial production of transgenic fish and shellfish raises issues of environmental safety. Concerns stem from the possibility transgenics that escape from production facilities might pose ecological or genetic hazards.
Environmental safety concerns must be considered on a case-bycase basis, focusing on the particular species, culture system, and ecosystem at issue. The few empirical studies assessing the environmental safety of transgenic fishes suggest some risk may be realized. No risk assessment studies have yet considered transgenic shellfishes.
Public policy & regulations
Public policy concerning commercialization of transgenic fish and shellfish is not well developed. However authorized, fisheries or marine conservation agencies should review proposed activities concerning commercial production of transgenic fish and shellfish.
Descriptions of proposed activities should discuss possible risks and proposed risk management measures. The petition for the permit should be open to public scrutiny and comment, both oral and written. Granting of a permit may be conditional on the producer demonstrating the effectiveness of confinement and agreeing to share data relevant to risk assessment and risk management.
Confinement
Commercial production of transgenic fish and shellfish should be permitted only in wellconfined systems. At least at the outset, commercial production of transgenic fish and shellfish might be limited to indoor, recirculating aquaculture systems. A particularly well-designed program of confinement measures would be needed for production in outdoor systems, such as ponds or raceways. Reproductive confinement should be required, for example, by producing triploid or monosex stocks.
A practical means for promoting effective confinement would be to require adherence to the Performance Standards for Safely Conducting Research with Genetically Modified Fish and Shellfish (http://www.nbiap.vt.edu/perfstands). Public policy regarding commercial production of genetically modified fish and shellfish must be revisited frequently, so that it evolves with advances in the understanding of risks posed.
Public acceptance
Ongoing controversies regarding products from transgenic crop plants suggest consumer acceptance is the key factor determining the success or failure of commercialization of genetically modified (GM) foods. Most consumers (65 percent) express confidence in the ability of government agencies to oversee food safety aspects of biotechnology. A large majority (85 percent) favor explicit labeling of GM food products.
Producers of GM foods, however, have resisted labeling their products as containing GM components. Survey results also show that for consumers to favor GM over conventional foods, they will have to perceive consumer benefits from unique product qualities (such as improved nutritional value) or lower price. So far, the benefits of GM foods have accrued only to producers.
How transgenic fishes are produced
Several steps are needed to produce a line of transgenic fish. Agene construct must be prepared that contains the DNA encoding of the protein to be expressed in the host. The construct also contains DNA sequences that regulate expression of the introduced gene in the host. These sequences determine the level of expression, the tissues in which the gene is expressed, and the developmental stages when the gene is expressed in the host.
The gene construct must be inserted into the DNA of the species of interest. This most often is accomplished by microinjection of a solution containing the gene construct into newly fertilized eggs of the species of interest.
A range of molecular analyses will be employed to identify individuals into whom the introduced gene has been incorporated and expressed. Some, but not all, of the original generation transgenic fish will transmit the introduced gene to their descendants. Their offspring will be screened to determine whether the introduced gene was transmitted.
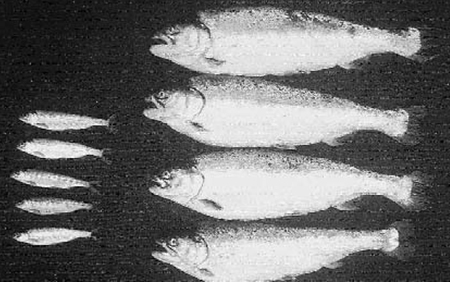
Families in which the gene was transmitted will be performance tested to determine whether expression of the introduced gene affected the targeted trait as hoped. For example, expression of an introduced growth hormone gene accelerated growth of transgenic coho salmon (right) relative to that of non-transgenic siblings (left).
Members of high-performance families will be mated to produce a true-breeding transgenic line, providing the foundation stock for commercialization. Biotechnology firms generally will market their product by selling fertilized eggs or fry to fish farmers.
Conclusion
Successful commercialization of transgenic fish and shellfish will require public confidence that food safety and environmental risks are thoroughly addressed, and that key consumer and societal interests are respected.
(Editor’s Note: This article was originally published in the December 2000 print edition of the Global Aquaculture Advocate.)
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Author
-
Eric M. Hallerman, Ph.D.
Department of Fisheries and Wildlife Sciences
Virginia Polytechnic Institute and State University
Blacksburg, Virginia USA
Tagged With
Related Posts

Innovation & Investment
AquaBounty, with new RAS facility, hopes to win public support for GM salmon
Ron Stotish, CEO of AquaBounty Technologies, believes genetically modified salmon is no threat to its opponents and the outlook for AquAdvantage is good. With its purchase of the Bell Fish Co. RAS facility, commercialization will soon commence.

Innovation & Investment
Aquaculture Exchange: Ron Stotish, AquaBounty Technologies
Ron Stotish, CEO of AquaBounty Technologies, discusses being the first to produce a genetically modified (GM) farmed salmon deemed safe for consumption, the controversy surrounding his company's product and the potential of biotechnology.

Intelligence
A brief look at genetically modified salmon
If approved by FDA, fast-growing genetically modified salmon will provide a safe and nutritious product similar to other farmed Atlantic salmon.

Intelligence
GM salmon and the FDA: 10 takeaways
After a 20-year process, a genetically modified fish earned U.S. Food and Drug Administration approval, reigniting one of the seafood industry’s most intriguing controversies. Here are 10 key downloads from the groundbreaking decision over AquAdvantage salmon.


