REPROSEED supports bivalve hatchery development in Europe
Although academic knowledge is reported in scientific publications, practical progress in bivalve aquaculture has relied largely on empirical approaches. This situation is particularly acute for bivalve hatchery/nurseries, as their activities have developed only over the last 30 years, and the limiting factors have seldom been considered systematically.
Such hurdles are often species-specific and concern different stages of the mollusc biological cycle. They mainly involve broodstock management and gamete quality, and the reliability of larval-rearing methods. Metamorphosis synchronization and improvement of settlement success have been challenging, as well as the quality of seed in terms of immunity, genetic diversity and sanitary status.
REPROSEED project
Advances in these areas will undoubtedly lead to better hatchery methodology that will improve the reliability of spat production. Therefore, the REPROSEED – REsearch to improve PROduction of SEED – project was established in 2010 to link academic and applied approaches to examine complex biological processes and provide innovative technology that will improve bivalve seed production in Europe in terms of reliability, quality and cost.
As the project moves toward a conclusion in 2014, a further objective is the transfer of knowledge among sectors on the culture techniques for different bivalve species. Emerging species for which hatchery-based production of seed is still limited in Europe, such as European clams, king scallops and blue mussels, will benefit from advances with more established species, such as Pacific oysters.
REPROSEED is being conducted by a consortium of nine research or technology and development institutes, two enterprise hatcheries and a professional association from seven European countries. It is financed by the European Union and coordinated by Ifremer.
Objectives
The main objectives of the REPROSEED project concern the reliability and capacity of hatcheries to respond to the increasing demand for mollusc seed that has arisen due to the unreliability of natural spatfall caused by fluctuating environmental conditions in the wild. Moreover, using optimized hatchery/nursery-rearing techniques, mollusc genetic improvement through selective breeding and/or polyploidization could be developed under controlled conditions. Finally, continuous interaction with end users in this project will facilitate the transfer of knowledge and new technology, and thus the development of more-efficient shellfish hatcheries in Europe.
Targeted species
Four bivalve species are being targeted due to their economic importance in Europe and the different biological challenges they represent with varied reproduction, settlement and sensitivity to bacteria at the larval stage. Overall hatchery seed production costs are expected to be reduced through the project.
REPROSEED centers its research on Pacific oyster and three emerging species in hatchery production. The Pacific oyster (Crassostrea gigas) is the commercial mollusc species for which scientific knowledge is the most advanced, and it has the most commercial seed supply activity in Europe.
The king scallop (Pecten maximus) is presently produced in hatcheries in France and Norway, although not all of its rearing difficulties have been overcome. Due to its high susceptibility to bacteria, it is an excellent model for developing specific research in the field of prophylaxis, which will reduce the need for antibiotics.
The blue mussel (Mytilus edulis and closely related M. galloprovincialis) are of major importance for the European shellfish industry. There is increasing demand for seed availability in the Netherlands, Spain and France, where hatcheries are expected to play a significant role in the near future.
For the European clam (Ruditapes decussatus) only a very small amount of technological development has been made compared with that for the Manila clam (R. philippinarum).
The scientific work plan is organized into four work packages closely linked to the main bivalve biological stages: maturation/reproduction, larval stage, metamorphosis and postlarval stage. Two cross-cutting axes are devoted to knowledge improvement in genomics and microbiology.
Initial results
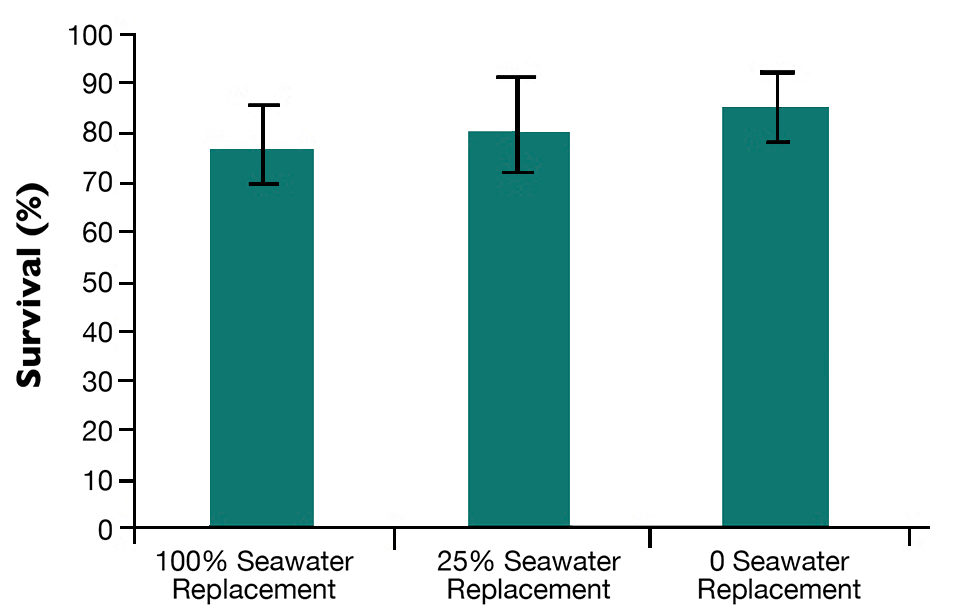
The first results from REPROSEED mainly concerned larval and spat rearing in recirculating aquaculture systems (RAS). Such systems seem promising for oysters. The RAS is also well adapted to scallop spat. Survival rates are shown in Fig. 1.
The bacterial analyses performed during experiments in RAS did not show any higher bacterial concentrations in seawater and larvae than in conventional rearing systems, even with low seawater renewal. Nevertheless, they revealed high vibrio levels in biofilms of scallop larvae RAS, apparently without a negative impact on scallop survival.
The outdoor mass production of microalgae in paddlewheel raceways was tested using alternative media that could reduce production costs. In a comparison with the use of standard Conway medium as a control, diatoms grew correctly on a basic nutrient formula, while the continuous cultures could be maintained for more than 10 days.
The preconditioning of oysters greatly influences their reproductive effort in germinal cell proliferation and storage. For gamete quality assessment, the physical parameters of gamete size and spermatozoa movement were measured, as well as adenosine triphosphate, protein and lipid contents. Gene expression was examined by real-time polymerase chain reaction testing.
The important parameters of protein content and motility were relatively well correlated with egg quality, while insulin gene expression and hatching rate were significantly correlated. In gel proteomic analysis, several proteins differentiated good-quality oocytes from bad-quality oocytes. Their identification is in progress.
Spawning, genomic resources
Different inducers of metamorphosis, including KCL, ammonium chloride, epinephrine and gamma-aminobutryic acid were tested at different concentrations and exposure times on mussel and clam larvae. They did not induce a systematic increase of metamorphosis rates, and their utility was questionable. Spawning inducers are also being sought, notably among the neuropeptides and gonadal peptides of C. gigas involved in spawning.
The acquisition of new genomic resources in scallops, mussels and clams are almost finished. Tissues from eggs, larvae at different development stages, postlarvae, juveniles and broodstock have been collected to establish mRNA libraries. Thereafter, oligo-microarrays will be created to compare extreme conditions at different bivalve life stages. The most interesting genes will be used to focus on adaptive responses to stress and studied by real-time polymerase chain reaction.

Outlook
The REPROSEED project has been seen in a positive light by the industry. A survey designed to understand hatchery capacities and take into account their demands has aided the relationship with end users.
Technology such as that perfected in RAS for oyster larvae and postlarvae will soon be extended to clams and mussels. The next step will be to implement the technology at the pilot scale in a commercial hatchery.
The mass algal production in paddlewheel raceways still requires improvement in terms of algal cell concentration and culture duration, but the recent results obtained with diatoms are promising. The advances in nutrition, reproduction, larval rearing and metamorphosis should allow the project to develop and stabilize the production of European clam seed, mainly in Portugal.
The numerous data generated by genomic and proteomic analyses will help improve the understanding of the physiological effects of different rearing conditions, including diet, stress and physico-chemical parameters, and assess the quality of the gametes, larvae and seed. The transfer to commercial hatcheries should be initiated at the end of this year with a first meeting between the representatives of the REPROSEED participants and professionals of the shellfish hatchery industry.
(Editor’s Note: This article was originally published in the March/April 2013 print edition of the Global Aquaculture Advocate.)
Authors
-
Jean-Louis Nicolas, Ph.D.
Laboratoire de Physiologie des Invertébrés
PFOM-LEMAR
IFREMER, Centre Bretagne
C.S. 10070
29280 Plouzané, France -
René Robert, Ph.D.
Laboratoire de Physiologie des Invertébrés
PFOM-LEMAR
IFREMER, Centre Bretagne
C.S. 10070
29280 Plouzané, France -
Piere Boudry, Ph.D.
Laboratoire de Physiologie des Invertébrés
PFOM-LEMAR
IFREMER, Centre Bretagne
C.S. 10070
29280 Plouzané, France
Tagged With
Related Posts

Responsibility
Assessing the carbon footprint of aquaculture
A carbon footprint is an estimate of the total carbon emissions resulting from the production, use and disposal of a product or service. Carbon footprints for aquaculture products result mainly from the use of manufactured feed and mechanical aeration.

Responsibility
Atmospheric pollution affects water quality
Acid rain typically does not heavily affect aquaculture operations, and application of agricultural limestone can buffer water against the impacts of acid rain at facilities that use stream water.

Responsibility
Can sustainable mariculture match agriculture’s output?
Global, sustainable mariculture production, developed on a massive, sustainable scale and using just a small fraction of the world’s oceanic areas, could eventually match the output of land-based agriculture production. Scale and international law considerations require the involvement of many stakeholders, including national governments and international organizations.

Intelligence
Behold the nutritious oyster
Oysters provide important, natural filtration of water and are an important component of many healthy coastal ecosystems because their active filtering can help improve and maintain water quality. For many coastal communities, oysters are an important food resource and excellent sources of protein and amino acids, zinc, selenium, iron and B-vitamins.


