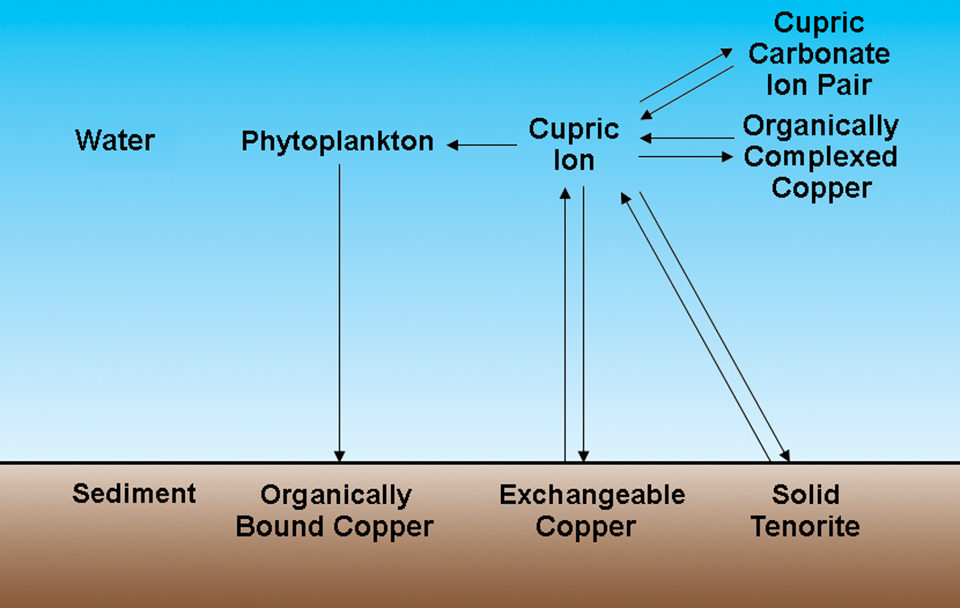
Cupric ions have several possible fates in aquaculture ponds

Copper is a minor mineral component in natural waters that usually occurs at concentrations of 0.002 to 0.004 milligrams per liter in sea water and 0.005 to 0.020 milligrams per liter in fresh water. At low concentrations, copper is a micronutrient required by all plants, but above 0.05 milligrams per liter it can be toxic to algae. Copper also is an essential nutrient for animals at low concentration and a toxin at higher concentration.
Phytoplankton, and especially blue-green algae, are highly sensitive to copper, so this metal is used widely as an algicide in water supply lakes, swimming pools, cooling towers, sport fish and aquaculture ponds, and other aquatic systems. The traditional copper formulation used as an algicide is copper sulfate. It is a blue, granular substance that is also called blue vitriol or bluestone. Copper sulfate contains 26 percent elemental copper by weight.
Copper chemistry
When applied to water, copper sulfate quickly dissolves to yield cupric ions and sulfate. Cupric ions have several possible fates in aquaculture ponds (Fig. 1). Depending upon the pH, a variable amount of cupric ion remains in solution. Assuming that the mineral controlling the solubility of copper is tenorite, the equilibrium concentration of cupric ions is 0.02800 milligrams per liter at pH 7 and 0.00028 milligrams per liter at pH 8 (Table 1).
Cupric ions react with carbonate ions to form a soluble cupric carbonate ion pair. In aquaculture pond water, the amount of copper in the ion pair will greatly exceed the cupric ion concentration. Cupric ions form soluble complexes with naturally occurring organic acids in water. There also may be more copper in the complexed form, often called the chelated form, than in cupric ions.
Algicide use
Three processes act to remove copper from water. Water plants absorb copper, and when they die, copper settles to pond bottoms in dead organic matter. Cupric ions precipitate rapidly as cupric oxide and can be adsorbed on colloidal clay and organic matter in bottom soil through cation exchange processes.
The cupric ion is a form of copper toxic to plants, but it is only slightly less toxic to aquatic animals. The concentration considered acceptable for phytoplankton control ranges 0.06 to 0.50 mg copper per liter of water. The maximum recommended concentration of copper for fish is usually 0.02 milligrams per liter in acidic, low-alkalinity water and 0.2 milligrams per liter in water with high alkalinity and pH.
The solubility of cupric ions is greatest at low pH and decreases rapidly with increasing pH (Table 1). This poses two problems for the use of copper sulfate as an algicide. At low pH, the relatively high concentration of cupric ions favors high toxicity to phytoplankton, but there is risk of harming the culture species. At higher pH, there is less danger in harming the culture species, but it is more difficult to achieve a great enough cupric ion concentration to control phytoplankton.
Boyd, Concentration of cupric ions, Table 1
| pH | Cupric Ion Concentration (mg/l) |
|---|
pH | Cupric Ion Concentration (mg/l) |
|---|---|
| 5 | 28 |
| 6 | 2.8 |
| 7 | 0.028 |
| 8 | 0.00028 |
| 9 | 0.0000028 |
Chelated copper products
Copper ions can be chelated with organic compounds such as triethanol amine, ethylene diami-netetraacetic acid, lignin sulfonate, or citric acid. The benefit of treating water with chelated copper products is that the concentration of dissolved copper can be maintained at an elevated level for several days, even in waters with high pH and alkalinity.
Chelated copper is toxic to phytoplankton, but not nearly as toxic as cupric ions to aquatic animals. Thus, chelated copper products for phytoplankton control can be used in acidic, low-alkalinity waters without fear of harming the culture species. Moreover, in high-alkalinity water of greater pH, an effective copper concentration can be achieved more easily by using chelated copper products. The main disadvantage of chelated copper compounds is their high cost, which is usually several times greater than copper sulfate per unit weight of copper.
In high-alkalinity waters with pH of 7.5 or more, relatively large applications of copper sulfate can be made without endangering culture species. In fish culture, the usual copper sulfate dose is 0.01 of the total alkalinity. For example, if total alkalinity is 135 milligrams per liter as calcium carbonate, the copper sulfate dose should be 1.35 milligrams per liter.
It is more economical in moderate- to high-alkalinity waters to use copper sulfate and re-treat at frequent intervals, if necessary, to effect phytoplankton control than to use chelated copper products. In acidic, low-alkalinity water, the concentration of copper sulfate needed for phytoplankton control can exceed the tolerance of the culture species to copper. In this situation, chelated copper products should be used.
Use in catfish farming
In channel catfish farming, copper sulfate treatments are a widely used means of thinning blue-green algae before harvest for the purpose of avoiding off-flavors and potential rejection of the fish by processing plants. Many catfish ponds in the southeastern United States have been treated with copper sulfate several times per year for 10 or more years.
The copper quickly disappears from the pond water, and copper concentrations return to pretreatment levels within 72 hours (Fig. 2). Copper accumulates in the sediments of ponds, but because of its low solubility at pH 6 or above, contamination of pond waters by copper released from sediment has not occurred.
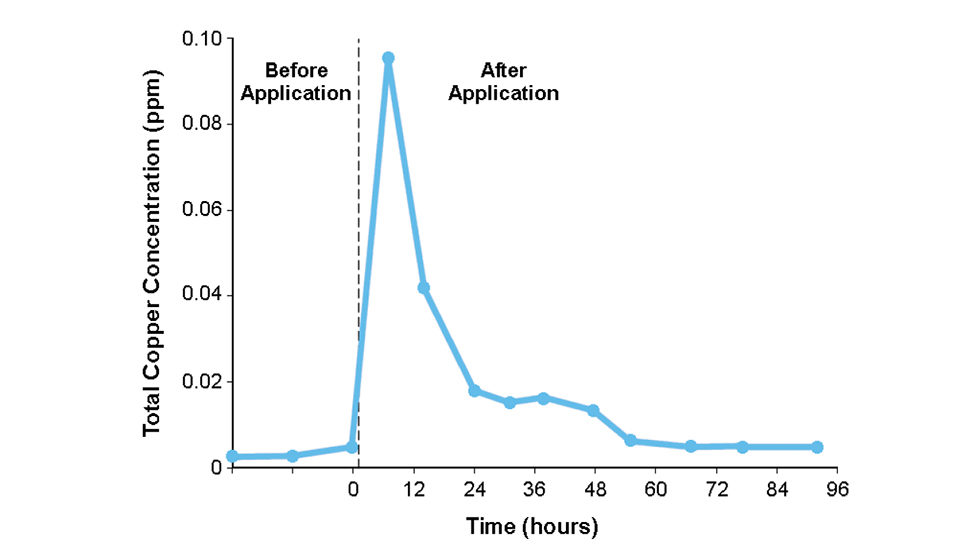
Catfish farming is done in areas where pond bottom soils are neutral or slightly alkaline, and a different response might be obtained in acidic ponds. If water is not discharged from ponds for three days after treatment, and treatments are not made before large rainfalls that might cause overflow, the use of copper sulfate in aquaculture ponds should not result in environmental contamination.
Use in shrimp culture
Copper algicides have not been used widely in shrimp aquaculture, but should be as effective in brackish water shrimp ponds as in fresh water fish ponds. Brackish waters generally have alkalinity values above 100 milligrams per liter, so copper sulfate could be safely applied.
Application
Copper sulfate can be applied by several methods. The most common are broadcasting crystals over pond surfaces, dissolving the algicide in water for spraying over pond surfaces, and suspending copper sulfate in porous bags in front of aerators or where inflow enters ponds.
Copper sulfate also can be used to treat mat-forming algae around pond edges. The usual method is to broadcast copper sulfate crystal over the algal mats.
Chelated copper algicides can be mixed with water and sprayed over pond surfaces. Also, some workers mix chelated copper algicides with water and drain the solution from a boat-mounted tank into the turbulence caused by the propeller of an outboard motor while driving the boat over the pond surface.
When treating ponds with copper compounds and copper sulfate in particular, it is critical to have a reliable estimate of pond volume. Massive, copper-related fish kills in channel catfish ponds have resulted from mistakes in dose calculations. It also is important to limit the amount of phytoplankton killed at one time to avoid low dissolved-oxygen concentrations. This can be achieved by treating only one part of a pond at a time.
(Editor’s Note: This article was originally published in the April 2005 print edition of the Global Aquaculture Advocate.)
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Author
-

Claude E. Boyd, Ph.D.
Department of Fisheries and Allied Aquacultures
Auburn University
Alabama 36849 USA
Tagged With
Related Posts

Responsibility
Calculating chemical treatments for aquaculture production
In calculating chemical treatments to apply to ponds, one must adjust for the percentage of the active ingredients in products. Adjustments for both active ingredient content and specific gravity must be made for liquid products.

Health & Welfare
Chemical treatment costs reduced with in-pond raceway systems
By dividing ponds into smaller, more controllable areas, IPRS can substantially reduce chemical treatment costs, improve treatment delivery and allow new treatments that are cost-prohibitive in traditional pond settings.

Health & Welfare
Common diseases of pangasius catfish farmed in Vietnam
As with other species, varied health problems – and treatments – related to parasites and bacteria have been encountered in pangasius catfish.

Intelligence
Considerations for tilapia farming in saltwater environments
Tilapias are farmed in a variety of production systems, but mostly in freshwater and low-salinity waters. But tilapias are an excellent candidate for aquaculture in brackish- and seawater because they can tolerate a wide range of water salinity.

