Chalk, marl, crushed seashells and wood ash are not as effective as calcite

Shrimp and fish farmers frequently apply liming materials to ponds to increase the pH of bottom soils and elevate alkalinity and hardness in water. These chemical changes improve conditions for microbial activity and benthic animals; increase the availability of carbon dioxide, phosphorus, and other nutrients; enhance phytoplankton growth; and improve the survival and growth of the aquaculture crop.
Confusion about the properties of liming materials, however, often results in these materials being used incorrectly. And, of course, if soil or water are not acidic, treatment with agricultural limestone will be of little or no benefit.
Common forms
The most common liming materials are agricultural limestone, burnt lime or quick lime, and hydrated lime or slaked lime. All of these products are made from limestone. Limestone is a relatively soft rock composed of calcium carbonate (CaCO3 or calcite), calcium magnesium carbonate (CaCO3·MgCO3 or dolomite), or a mixture of these carbonates.
Solubility
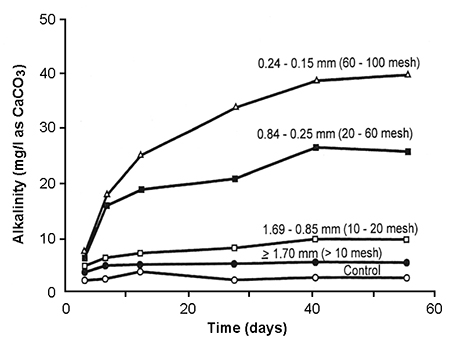
Like most rocks, limestone in raw form is not highly soluble. By pulverizing it into fine particles, the surface area:mass ratio is increased to enhance solubility, as measured by increase in alkalinity (Fig. 1). In good-quality agricultural limestone, most particles will pass a 60-mesh sieve (0.25-mm openings). In high-quality agricultural limestone, most particles will pass a 100-mesh sieve (0.15-mm openings).
Color
The color of agricultural limestone is variable. Most samples are a shade of white, but can also appear red, yellow, or brown.
Other liming materials
Other materials that have been used for liming include chalk (soft limestone), marl (loose carbonate deposits often mixed with clay), crushed seashells, and wood ash. Most are not as effective as calcite, and some of their byproducts can contain harmful levels of heavy metals or other undesirable contaminants.
Chemical reactions
When agricultural limestone reacts with acidity, the acidity is neutralized and calcium carbonate is transformed to calcium ions, carbon dioxide, and water. Magnesium carbonate in agricultural limestone reacts with acidity in a similar manner, but magnesium ions instead of calcium ions are released.
The most important reaction of agricultural limestone in water is with carbon dioxide. This reaction results in dissolution of the calcium carbonate and an increase in the calcium and bicarbonate concentrations in solution. Magnesium carbonate reacts in a similar manner, but magnesium and bicarbonate result.
Water hardness
The total hardness of water is a measure of the calcium and magnesium concentration expressed as equivalent calcium carbonate. For example, suppose a water contains 5 milligrams per liter calcium and 1 milligrams per liter magnesium. The total hardness in equivalent calcium carbonate can be determined as:

In practice, total hardness can be measured directly by titration of calcium and magnesium with ethylenediamine-tetraacetic acid (EDTA).
Alkalinity
The total alkalinity of water results from the titratable bases expressed in terms of equivalent calcium carbonate. Bicarbonate usually is the main base in water. Alkalinity is measured by titration of a water sample to pH 4.5 with standard acid and estimation of the calcium carbonate equivalence of the amount of acid used.
Limestone and water
Application of agricultural limestone to water normally increases total alkalinity and hardness by roughly equal amounts. However, the solubility of calcium carbonate in water in equilibrium with normal atmospheric concentrations of carbon dioxide is about 60 milligrams per liter. In ponds, there usually is more carbon dioxide available from organic matter decomposition, and calcium carbonate is more soluble. Nevertheless, if pond waters contain more than 80 to 100 milligrams per liter alkalinity, agricultural limestone usually will not dissolve.
Limestone and soil
Agricultural limestone reacts in soil much the same way it does in water. However, the calcium and magnesium ions that result from its reaction with hydrogen ions or carbon dioxide exchange for aluminum and iron ions absorbed on soil colloids. This reduces the acidity of the soil and causes its pH to increase. The pH of soil cannot be increased above 8.3 by application of agricultural limestone.
Liming effects
The primary effects of agricultural limestone applications in aquaculture systems are to decrease acidity and increase the pH, hardness, and alkalinity of acidic soils and water. These chemical changes improve conditions for microbial activity and benthic animals; increase the availability of carbon dioxide, phosphorus, and other nutrients; enhance phytoplankton growth; and improve the survival and growth of the aquaculture crop. Of course, if soil or water are not acidic, treatment with agricultural limestone will be of little or no benefit.
Burnt and hydrated lime
Burnt lime is made by burning limestone in a furnace at high temperature. Hydrated lime is made by treating burnt lime with water. Both burnt lime and hydrated lime usually are fine, white powders. When put into ponds, calcium oxide reacts with water and forms calcium hydroxide, which neutralizes acidity and causes pH to rise. Calcium hydroxide also can react with carbon dioxide.
When added to water, burnt lime and hydrated lime have the same effects as agricultural limestone, but initially cause a much higher pH. Burnt and hydrated limes should not normally be applied to pond water during the culture period to prevent potentially harmful high pH levels. These two materials, however, can be applied to the bottoms of empty ponds at 1,000 to 2,000 kilograms per hectare to raise pH and kill pathogens and other unwanted organisms.
Neutralizing values
The strength of liming materials is reported as a neutralizing value, with the value of pure calcium carbonate established as 100 percent. Based on this system, the neutralizing values of pure compounds used as liming material are:
Calcite 100 percent
Dolomite 108 percent
Hydrated Lime 135 percent
Burnt Lime 178 percent
Agricultural limestone made from pure dolomite is 1.08 times as strong in neutralizing acidity as that made from calcite. Burnt lime and hydrated lime are, respectively, 1.78 and 1.35 times as strong in neutralizing acidity as agricultural limestone made from calcite. Of course, individual samples of liming materials will vary because of impurities.
(Editor’s Note: This article was originally published in the August 2002 print edition of the Global Aquaculture Advocate.)
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Author
-

Claude E. Boyd, Ph.D.
Professor, Department of Fisheries and Allied Aquacultures
International Center for Aquaculture and Aquatic Environments
Auburn University
Auburn, Alabama 36849 USA
Tagged With
Related Posts

Responsibility
A review of water quality improvement products
Prof. Boyd examines products used by aquafarmers to improve water quality and conditions in their ponds and discusses their efficacy.

Responsibility
Constantly changing pH unavoidable, completely normal
Prof. Claude Boyd discusses the importance of pH for farmed fish and shellfish, the normal and natural fluctuations and how aquaculture systems can manage it.

Responsibility
Factors affecting efficiency of commercial fertilizers in aquaculture
Intensification of pond aquaculture involves the use of commercial fertilizers such as urea and triple superphosphate to stimulate phytoplankton blooms. There is no objective way of determining the ideal fertilization rate for an individual pond.

Responsibility
Addressing safety in Latin America’s tilapia supply chain
Over the last decade, the experience gained by many tilapia farmers combined with proficient programs implemented by local governments have significantly improved tilapia production in various Latin American countries like Colombia, Mexico, Ecuador and other important tilapia producers in the region.

