Using well water for inland shrimp culture presents challenges

Although marine shrimp is the highest-value seafood product imported to the United States, the high cost of coastal land, user conflicts, and strict effluent requirements have, at least in part, limited the expansion of shrimp aquaculture in the U.S. Future increases are likely to come from inland production, which entails the use of ground waters with ionic composition vastly different from seawater.
Currently, shrimp are being farmed in freshwater and ground waters of less than 10 ppt salinity in at least four U.S. states: Florida, Texas, Arizona, and Alabama. Information from these farms and researchers at the Harbor Branch Oceanographic Institution (HBOI) indicates adequate growth rates and acceptable production of the Pacific white shrimp, (Litopenaeus vannamei). This marine shrimp tolerates a wide range of salinity and is capable of survival and growth at salinities as low as 0.5 ppt, which is technically considered freshwater.
Saline groundwater
There is saline well water under two-thirds of the conterminous United States. Its salinity derives from a number of sources, including salt domes, geologically old water trapped from the last ice age, deep geothermal wells, and saltwater intrusion from coastal areas. Unfortunately, these waters vary in individual ion concentrations and total salinity, which also differs between locations and well depths. Farmers wishing to explore shrimp culture in freshwater have had to conduct their own pilot-scale trials.
Challenges for development
Using well water for inland marine shrimp culture presents challenges. Information on the essential environmental ions involved in shrimp osmo-regulation (Na+, Cl–, Ca2+, Mg2+, and K+) and the minimum concentrations necessary for survival and growth of marine shrimp cultured in freshwater is lacking. Determination of the ion concentrations required by L. vannamei will aid in determining potential inland sources of water suitable for intensive shrimp culture.
Survival studies
HBOI conducted short-term survival experiments on L. vannamei postlarvae using five essential ions:
Na+, Cl–, Mg2+, Ca2+, and K+. Separate monovalent (Na+ per K+) and divalent (Mg2+per Ca2+) ion solutions were prepared using chloride-based chemicals added to distilled water.
The monovalent and divalent ion ratios in the two solutions were equal to those found in full-strength seawater. These monovalent and divalent solutions were then combined volumetrically to produce various ion concentrations (Table 1).
McGraw, Mean 48-hour survival of PL18 L. vannamei in various ion, Table 1
| Ion Treatment | Ca2+ | Mg2+ | Na+ | K+ | Cl- | 48-hour % survival |
|---|
Ion Treatment | Ca2+ | Mg2+ | Na+ | K+ | Cl- | 48-hour % survival |
|---|---|---|---|---|---|---|
| 100% Divalent* | 67 | 210 | 0 | 0 | 738 | 0 |
| 100% Monovalent* | 0 | 0 | 383 | 14 | 60 | 37 |
| 75% Div.: 25% Mon. | 50 | 158 | 96 | 4 | 706 | 50 |
| 50% Div.: 50% Mon. | 33 | 105 | 191 | 7 | 669 | 63 |
| 25% Div.: 75% Mon. | 17 | 53 | 287 | 11 | 638 | 67 |
| HBOI well water | 44 | 31 | 181 | 10 | 280 | 73 |
Results showed the absence of monovalent or divalent ions in treatment solutions produced survival rates below 37 percent. In general, ion treatments with a higher monovalent percentage, but not only monovalent ions, had higher shrimp survivals.
Growth studies
Although this and other short-term survival experiments conducted at HBOI have shown that various freshwater ion concentrations are acceptable, growth in these solutions may be negligible. Therefore, a growth experiment incorporating an ion ratio scheme similar to the above was conducted.
L. vannamei were cultured in synthetic freshwater solutions with less than 1 ppt salinity. Postlarval shrimp (PL15) were placed in 4-l plastic containers half filled with the various treatment and control waters. Adequate aeration was provided by diffused air. Shrimp were offered a prepared feed 3 times per day based on body mass. A portion of all ion solutions was replaced daily.
Survival and weight
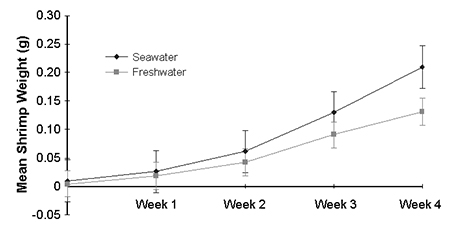
At the start of week 2, mean growth for all ion treatments was 62 percent, as compared to the HBOI freshwater and seawater controls. By the end of week 3, shrimp in treatment solutions had 100 percent mortality, whereas shrimp in seawater and HBOI freshwater were still alive. Shrimp survival did not correlate with any ion treatment or total salinity.
After four weeks, shrimp in full-strength seawater were significantly heavier than those in HBOI freshwater (Fig. 1). However, this weight difference may have been caused by poor water quality rather than a salinity difference.
Water quality
Mean total ammonia-nitrogen levels for all treatments and controls ranged 0.65 to 2.05 ppm (0.026 to 0.082 ppm nonionized ammonia). Nitrite levels ranged 0.07 to 0.7 ppm. Water exchange rates were increased as ammonia and nitrite levels increased in an attempt to preserve water quality.
Low levels of ammonia and nitrite may act synergistically with osmotic stress and molting to cause high shrimp mortality in these solutions. This has been reported for other shrimp species. Nitrogenous waste products are excreted from shrimp gills via ion exchange reactions. Therefore, osmoreg–ulation and waste excretion are related.
Conclusion
Inland production of shrimp using groundwater can provide an alternative to traditional coastal aquaculture in the United States. Its future, however, may hinge on a greater understanding of the environmental ions essential to shrimp growth, survival and osmoregulation in freshwater.
In studies of L. vannamei, researchers at Harbor Branch Oceanographic Institution found that shrimp exposed to ion solutions with relatively higher monovalent percentages yielded higher shrimp survivals. Shrimp raised in seawater, however, reflected much greater growth and survival than those in freshwater.
Minor elements found in seawater, but not the experimental ion treatments, may be necessary for successful shrimp aquaculture in freshwater. Future experiments at HBOI will investigate the requirements and toxicity of trace elements in synthetic ion solutions.
(Editor’s Note: This article was originally published in the June 2002 print edition of the Global Aquaculture Advocate.)
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Authors
-
William J. McGraw, Ph.D.
Harbor Branch Oceanographic Institution, Inc.
Aquaculture Division
5600 U.S. Highway 1 North
Fort Pierce, Florida 34946 USA -
John Scarpa, Ph.D.
Harbor Branch Oceanographic Institution, Inc.
Aquaculture Division
5600 U.S. Highway 1 North
Fort Pierce, Florida 34946 USA
Tagged With
Related Posts

Intelligence
4-hexylresorcinol: sulfite-free control for melanosis in crustaceans
4-hexylresorcinol in a nonsulfite processing treatment against melanosis in crustaceans inhibits natural enzymes for shell hardening.

Aquafeeds
A look at corn distillers dried grains with solubles
Corn distillers dried grains with solubles are an economical source of energy, protein and digestible phosphorus to reduce feed costs and fishmeal usage.

Responsibility
Accuracy of custom water analyses varies
The reliability of trace element analyses reported by custom laboratories cannot be checked by simple techniques, and results may not always be accurate. One should check the reliability of major ion analyses by determining the charge balance and comparing the measured total ion concentration with the total ion concentration estimated from conductivity.

Responsibility
Anion-cation balance in water
The ionic composition of inland well water can vary from suitable to toxic to cultured animals. Reliable data on concentrations of major cations (calcium, magnesium, potassium, and sodium) and major anions (bicarbonate, sulfate, and chloride) is therefore important in the management of waters for inland shrimp farming.


