Industry needs more monoclonal antibody testing
Shrimp farming in Belize, Central America, was hard hit by Taura Syndrome Virus (TSV, now known as TSV serotype A) in 1996. In 1997, Helen Dixon reported a method to eradicate TSV developed by Nova Companies Ltd. After this method was presented at the World Aquaculture Society’s annual meeting in 1997, Belize cleaned up from the aftermath of TSV and stayed TSV-free until 2002.
Initial strain identification
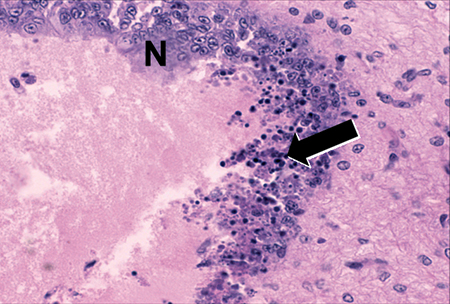
Industry reports of Taura Syndrome epizootics in a TSV-tolerant strain of (Penaeus stylirostris) and collected field evidence of Taura epizootics in (P. stylirostris) suggested a new TSV strain might have emerged since 1994. Investigation led to the discovery of the Sinaloa 1998 TSV isolate, which was characterized as serologically and genetically different than the Hawaiian 1994 TSV serotype A (TSV-A) published in GenBank.
Later industry reports of Taura syndrome epizootics of a magnitude not seen since the initial epizootics in (P. vannamei) suggested another new TSV strain might have emerged since TSV-B, the serotype in Sinaloa.
New strain suspected
In 2002, massive mortalities believed to be caused by TSV were reported in Belize shrimp aquaculture facilities. The University of Arizona Aquaculture Pathology Laboratory obtained an isolate of the agent from a naturally occurring epizootic of cultured P. vannamei from Belize to determine the cause of the mortalities. TSV was identified in the Belize samples using diagnostic methods set forth by the Office International des Epizooties.
reaction seen by the presence of blue-black precipitate in the TSV lesions.
Analysis was initially conducted using an injection and oral-feeding bioassay of frozen tissue with specific pathogen-free (SPF) P. vannamei Kona stock, reverse transcription polymerase chain reaction (RT-PCR) of frozen and ethanol-fixed tissues with TSV primers 9992 and 9195, hematoxylin and eosin (H & E) histopathology, in situ hybridization with TSV gene probes Q1 and P15, immunohistochemistry using TSV monoclonal antibody 1A1 (TSV MAb), and immuno-dot blot hybridization of purified virus using the TSV MAb.
Diagnostic profile
A bioassay of the Belize TSV tissue was used to determine pathogenicity in the SPF P. vannamei. TSV-A and TSV-B were bioassayed using the same population for comparison. The Belize TSV was the most pathogenic of the three virus strains, with only 35 percent survival by oral exposure and 25 percent survival by injection exposure (Table 1).
Erickson, Comparison of TSV strains administered orally and by injection, Table 1
| TSV Strain | Oral Challenge Survival | Injection Challenge Survival |
|---|
TSV Strain | Oral Challenge Survival | Injection Challenge Survival |
|---|---|---|
| TSV-A – Hawaii, USA | 40% | 40% |
| TSV-B – Sinaloa, Mexico | 35% | 35% |
| TSV-C – Belize, Central America | 35% | 25% |
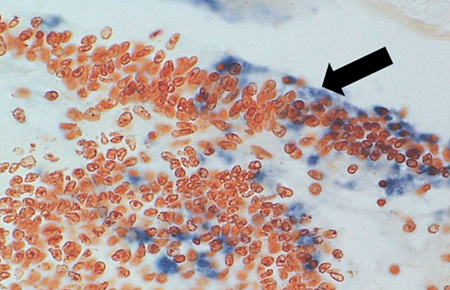
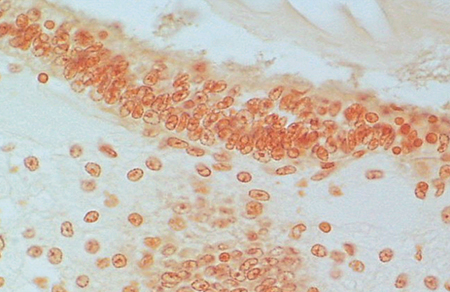
Diagnostic TSV RT-PCR fragments amplified from the Belize TSV tissue samples yielded the expected 231-bp TSV fragment (Fig. 1). Additionally, fixed tissues analyzed by routine H&E histopathology were found to present the TSV diagnostic lesions in the cuticular epithelium, as evidenced by the classic peppered or “buckshot” appearance of the tissue (Fig. 2). TSV was also detected by in situ hybridization with TSV gene probes (Fig. 3). However, Belize TSV was not detected using immunohistochemistry with TSV MAb in a serial section of the fixed tissue.
with TSV monoclonal antibody (MAb) 1A1.
Serial tissue section to that in Fig. 2. No reaction to TSV MAb 1A1.
Viruses subjected to fixation are in a denatured condition, whereas viruses purified from frozen tissue are still in a native state. To test if denaturing conditions played a role in the ability of the TSV MAb to recognize Belize TSV, the virus was purified from frozen tissue and then tested with the TSV MAb in an immuno-dot blot assay
The TSV MAb reacted only weakly with Belize TSV. The binding of the TSV MAb to the antigenic epitopes of the TSV strains was therefore different for each serotype.
Two diagnostic methods needed
Three strains of TSV, representing distinct TSV serotypes, have now been characterized: TSV-A, TSV-B, and TSV-C, the Belize 2002 strain (Table 2). However, only one TSV monoclonal antibody, TSV MAb 1A1, is commercially available.
Erickson, OIE-recognized diagnostic methods, Table 2
| Diagnostic Method | TSV-A | TSV-B | TSV-C |
|---|
Diagnostic Method | TSV-A | TSV-B | TSV-C |
|---|---|---|---|
| Gross Signs | ++ | ++ | ++ |
| Histopathology | +++ | +++ | +++ |
| Bioassay | + | + | + |
| Transmission electron microscopy | + | + | + |
| Antibody-based methods | |||
| Immunoblot (native virus) | +++ | – | + |
| IHC (denatured virus) | |||
| DNA Probes Q1, P15 (ISH) | +++ | +++ | +++ |
| RT-PCR (Diagnostic TSV primers 9992, 9195) | +++ | +++ | +++ |
– = negative/not able to detect specific TSV serotype.
Table 2. OIE-recognized diagnostic methods for detection of TSV.
When using MAb analysis, one must therefore consider that MAb-based results should not be relied on solely or as the only confirmatory test following H&E histopathology, and broodstock and postlarvae screening by MAb testing will not detect all TSV isolates. This could possibly lead to the further spread of TSV.
The shrimp pathology and diagnostic community is fully aware of the industry’s concerns and the need to develop an additional battery of TSV MAbs to use as a “cocktail” in MAb-based testing. TSV monoclonal antibody development is under way at the University of Arizona to produce additional antibodies that will react reliably with the additional TSV strains.
(Editor’s Note: This article was originally published in the December 2003 print edition of the Global Aquaculture Advocate.)
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Authors
-
Heidi S. Erickson, Ph.D.
Aquaculture Pathology Laboratory, University of Arizona
1117 E. Lowell, Building 90
Tucson, Arizona 85721 USA -
Bonnie T. Poulos
Aquaculture Pathology Laboratory, University of Arizona
1117 E. Lowell, Building 90
Tucson, Arizona 85721 USA -
Debbie Bradley-Dunlop
Aquaculture Pathology Laboratory, University of Arizona
1117 E. Lowell, Building 90
Tucson, Arizona 85721 USA -
Brenda White-Noble
Aquaculture Pathology Laboratory, University of Arizona
1117 E. Lowell, Building 90
Tucson, Arizona 85721 USA -

Donald V. Lightner, Ph.D.
Aquaculture Pathology Laboratory, University of Arizona
1117 E. Lowell, Building 90
Tucson, Arizona 85721 USA
Tagged With
Related Posts

Health & Welfare
A look at aquaculture genomics
Advances in genomics assist aquaculture science by deepening the understanding of adaptation, physiology and quantitative genetics.

Health & Welfare
A study of Zoea-2 Syndrome in hatcheries in India, part 1
Indian shrimp hatcheries have experienced larval mortality in the zoea-2 stage, with molt deterioration and resulting in heavy mortality. Authors investigated the problem holistically.

Health & Welfare
Biosecurity principles for sustainable production using SPF shrimp
Basic components of biosecurity include knowledge of diseases, adequate detection methods and the use of “clean” shrimp stocks.

Health & Welfare
Blue alternative: High Health introduces SPF blue shrimp to Thailand
Blue shrimp are very similar to Pacific white shrimp, and can be raised under similar conditions. Blues grow faster and tolerate lower water temperatures.


