Results of study show enhanced growth, reduced stress and immune system stimulation of cultured fish

Stimulating the immune system with feed additives to help prevent diseases and the use of therapeutants is becoming a widespread practice in fish culture. There are several downsides to this application method: First, additions to the feed require additional processing, which increases the costs. Second, the additive concentration in the feed is fixed and cannot be adjusted easily by the fish farmer depending on actual requirements. And third, because of feed competition, not all fish inside the same batch ingest the same amount of feed (additive), making the effects unpredictable: Too low concentrations can have no effects, too high can have adverse effects.
In fish, there is a second possible route of immunostimulant uptake: the gills. The large surface area of gills is not only necessary to guarantee sufficient oxygen uptake but is an entrance portal for microorganisms and foreign chemicals. Furthermore, the gill-associated lymphoid tissue plays an important role in the immune response and would be in direct contact with the bath stimulants. However, research information on stimulating the fish immune system via water treatment is still scarce, and long-term applications, similar to the use of immunostimulants in feed, has not yet been reported.
Humic substances (HS) are part of natural organic matter and represent up to 95 percent of dissolved organic matter (DOC) in aquatic ecosystems, with concentrations normally ranging from 0.5 mg carbon per liter (C/L) to 50 mg C/L. Their structure can differ greatly depending on their origin and seasonality and this chemical diversity is reflected by the heterogeneity of biological effects. The reported beneficial effects of HS, when used as feed additives, include growth stimulation, reduced mortality, accelerated recovery after diseases and stimulation of immune-related genes.
This article – adapted and summarized from the original publication (Lieke, T., et al., 2021. Phenol-rich fulvic acid as a water additive enhances growth, reduces stress, and stimulates the immune system of fish in aquaculture. Sci Rep 11, 174 (2021)) – investigated if humic substances, applied as a bath treatment, can stimulate the immune system of fish, and also which chemical structures characteristic of the humic substance applied could potentially be responsible for any biological effects observed.
Study setup
Eggs of rainbow trout (Oncorhynchus mykiss) were obtained from Uckermark-Fisch GmbH (Boitzenburg, Germany) and reared at the Leibniz-Institute of Freshwater Ecology and Inland Fisheries until fish reached the desired size. Fingerling rainbow trout (0 + years, 24.9 ± 2 grams, 13.3 ± 0.4 cm) were randomly distributed into nine tanks (40 liters, n = 30 per tank) in a flow-through system.
FulvoFeed is a commercial preparation of humic material procured from HuminTech GmbH, Grevenbroich, Germany. It was extracted from groundwater in wetland/bog-rich regions in northern parts of the Netherlands. After acclimatization, fish were exposed for four weeks to different (low: 0, 5; and high: 50 mg carbon, C/L) FulvoFeed concentrations added constantly by a peristaltic pump. The throughput rate of water and FulvoFeed were monitored twice per day to ensure constant exposure.
Fish were fed a commercial diet at 1.5 percent of their weight daily, and feeding was adjusted weekly to the calculated weight gain with an assumed feed conversion ratio of 1 (13.18 grams/fish in total over 28 days). During the experiment, temperature (16.5 ± 0.2 degrees-C), pH (8.5 ± 0.1), and dissolved oxygen (8.5 ± 0.5 mg/L) were monitored daily. Nitrite (0.3 ± 0.3 mg/L), nitrate (10.4 ± 2.0 mg/L) and ammonium (< 0.02 mg/L) were measured three times a week. Fish blood and tissue samples were collected before the start of the experiment, and two days and at the conclusion of the trial.
For detailed information on the experimental design and setup; fish husbandry; sample collections and various analyses; and statistical analyses, refer to the original publication.
Results and discussion
Our results, graphically summarized in Fig. 1, show that by adding FulvoFeed for 4 weeks to the rearing water of juvenile rainbow trout, we were able to increase fish length and weight gain by 5 and 10 percent, respectively, and to significantly improve feed conversion by around 16 percent in fish exposed to the high FulvoFeed concentration. These results are comparable to those obtained in another study using immunostimulants as feed additives. Against the background of the involvement of beneficial gut-bacteria in growth stimulation, it is intriguing that water supplementation with FulvoFeed resulted in stimulated growth parameters as well, as there is little direct contact between the gut and the FulvoFeed.
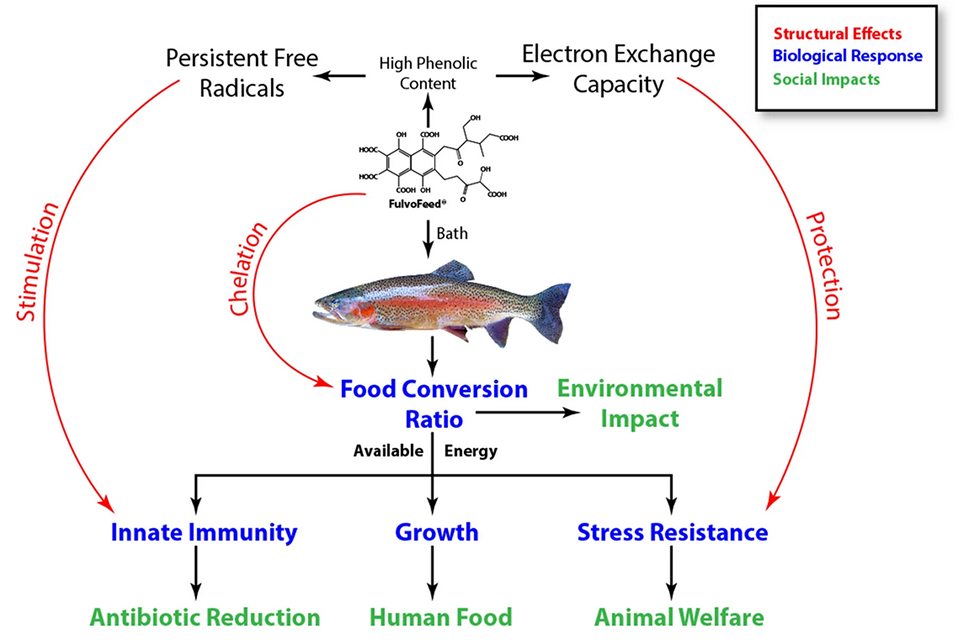
The significant reduction of the feed conversion ratio (FCR) indicates a better digestion efficiency and utilization of feed. Other researchers reported increased intestinal digestive enzyme activity in feed supplementation with fulvic acid, and chelation [a type of bonding of ions and molecules to metal ions] of mineral ions might promote the nutrient uptake and utilization of minerals in feed. This would eventually result in an increased amount of energy gained from the feed, which can then be allocated for the maintenance of steady physiological conditions, growth, and defense mechanisms.
An improvement of the FCR furthermore reduces the nutrient output (especially nitrogen and phosphorous) and the production of greenhouse gases, decreasing the impact on water bodies (if open systems are used) or wastewater production (in closed aquaculture recirculating systems) and the carbon footprint.
Stress, especially chronic one, and the allostatic load [wear and tear on the animal] imposed to cope with it, significantly affects the energy metabolism and enhances the production of extra energy supply from body resources. At the same time, stress leads to reduced or arrested feed intake and depressed immune system functions, impairing growth performance, and leaving the fish vulnerable to infections. Cortisol [hormone produced by many animals, and its release is regulated in response to stress and other factors] is most widely used to define stress levels. Exposure to FulvoFeed did not affect the cortisol concentration in blood of the experimental rainbow trout, indicating, that it did not exert any stress on the fish. In the laboratory, fish were kept under ideal conditions and external stress was kept at a minimum; it is therefore unlikely, that reduced stress during the exposure time accounts for the improvement detected in growth and FCR.
To mimic fish handling during culture operations, we exposed our fish to a strong acute stressor (netting and 30 seconds of air exposure). The cortisol response of fish conditioned with 50 mg C/L FulvoFeed was 24 percent lower than that of unexposed fish. It can be reasonably assumed that other stressors will be diminished as well. Further analyses, especially on molecular levels, are needed to work out the modes of action, but we were able to show that FulvoFeed addition to the rearing water helps to increase the growth of juvenile rainbow trout and reduce cortisol response to an acute stressor.
Our data also showed no apparent oxidative stress [a disturbance in the balance between the production of reactive oxygen species (free radicals: unstable atoms that can damage DNA) and antioxidant defenses] was exerted to the gills of our trout. This, together with other test results in our study, indicate that the applied FulvoFeed did not exert an apparent oxidative stress on the gills, and that its overall effect is protective.
The effects found with one humic substance cannot necessarily be transferred to another humic substance. The concentrations of FulvoFeed and the exposure duration used in our study did not induce negative effects like oxidative stress in rainbow trout as previously reported by other researchers for invertebrate species. However, whether or not the negative effects will appear at higher concentrations and/or longer exposure time has to be tested before any other long-term application can be recommended.
https://www.aquaculturealliance.org/advocate/efficacy-saturated-lipids-juvenile-california-yellowtail-feeds/
Phagocytes [cells that protect the body by ingesting harmful foreign particles, bacteria, and dead or dying cells] are an important part of the innate defense mechanisms [the body’s first line of defense against germs, providing an immediate or very rapid response against potential pathogens] and can reasonably be expected to increase the capacity to neutralize invasive microbes. Exposure to FulvoFeed increased the number of active phagocytes in the rainbow trouts’ head kidney [organ in bony fish analogous to the mammalian adrenal gland] by 83 and 124 percent, respectively. Furthermore, the number of particles digested by each phagocyte increased by 22 and 28 percent, respectively, and the potential killing activity increased significantly.
Altogether, this increases the microbiocidal capacity allowing the fish to defend themselves against pathogens. Similar effects were determined when using an oral application of immunostimulants. As chronic stress has been found to decrease the efficiency of macrophages (phagocytosis rate and ROS production),
Overall, results of our study showed that the FulvoFeed protective function works in two ways simultaneously: by increasing stress resistance and by stimulating the phagocytic activity and the potential killing activity of leucocytes [also known as white blood cells, are an important blood constituent and a key actor in the body’s immune system]. Defense mechanisms in rainbow trout are activated by bath exposure to FulvoFeed, which could help the fish protect themselves against diseases. However, more research including challenge experiments, is needed to evaluate if exposure to FulvoFeed helps to reduce the susceptibility of fish against specific pathogens.
Perspectives
Natural immunostimulants, commonly applied orally, can help improve animal growth and welfare while protecting from diseases by activating the host immune system. Our study results showed that stimulation over the gills is another pathway for cultured fish. Fulvic acids are part of aquatic ecosystems and bath application is a natural way to apply these immunostimulants, but their chemical structures must be determined before biological application.
We demonstrated that the addition of FulvoFeed to the culture water increased the growth of rainbow trout without adversely affecting the fish weight/ fat ratio, and also improved the feed conversion ratio (FCR). Also, the stress response was significantly lower in fish conditioned with FulvoFeed after netting and air exposure. Gills in treated fish, which are the entrance portal of viral and bacterial pathogens, had significantly improved defenses compared to non-treated fish.
The innate immunity was also significantly improved in terms of the increased activity and efficiency of leucocytes in the head kidney. The high aromatic contents of FulvoFeed, especially the phenolic moieties [part or functional group of a molecule], lead to a high EEC and protect against oxidative stress. At the same time, the PFRs in FulvoFeed exert mild stress, which stimulates the immune system.
Although protection against a specific pathogen has yet to be determined, FulvoFeed could help reduce the use of chemical therapeutants and prevent diseases in fish. Overall, we believe the addition of FulvoFeed to the rearing water of cultured fish is an easy and natural way to improve fish health and growth.
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Authors
-
Thora Lieke
Corresponding author and Ph.D. student
Department of Ecophysiology and Aquaculture, Leibniz-Institute of Freshwater Ecology and Inland Fisheries, Berlin, 12587, Germany; and Faculty of Life Sciences, Humboldt University of Berlin, Berlin, 10115, Germany -
Christian E. W. Steinberg, Ph.D.
Faculty of Life Sciences, Humboldt University of Berlin, Berlin, 10115, Germany; and Faculty of Environmental Science and Engineering, Kunming University of Science and Technology, Kunming, 650500, China
-
Bo Pan, Ph.D.
Faculty of Environmental Science and Engineering, Kunming University of Science and Technology, Kunming, 650500, China
-
Irina V. Perminova, Ph.D.
Lomonosov Moscow State University, Leninskie Gory, 119991, Moscow, Russia
-
Thomas Meinelt, Ph.D.
Department of Ecophysiology and Aquaculture, Leibniz-Institute of Freshwater Ecology and Inland Fisheries, Berlin, 12587, Germany
-
Dr. Klaus Knopf
Department of Ecophysiology and Aquaculture, Leibniz-Institute of Freshwater Ecology and Inland Fisheries, Berlin, 12587, Germany; and Faculty of Life Sciences, Humboldt University of Berlin, Berlin, 10115, Germany
-
Dr. Werner Kloas
Department of Ecophysiology and Aquaculture, Leibniz-Institute of Freshwater Ecology and Inland Fisheries, Berlin, 12587, Germany; and Faculty of Life Sciences, Humboldt University of Berlin, Berlin, 10115, Germany


