Study finds significant potential for TAN removal, disinfection

Fish production in land-based recirculating aquaculture systems (RAS) provides a number of well-known advantages. Some of the challenges are associated with the central biological processes, nitrification and denitrification. Both types of biofiltration are subjected to rearing conditions and require constant maintenance to ensure optimal performance. As nitrification relies on biotic conditions, start-up and colonization, unforeseen events – such as improper use of chemical disinfectant, abrupt change in salinity, extreme conditions (very low temperature) or suboptimal conditions (limited oxygen or alkalinity) – may lead to an undesirable accumulation of ammonia.
Similarly, insufficient nitrate removal may occur if conditions are not met; for example, large flows and lack of (costly) easy degradable carbon sources. On top of that, potential risks of highly toxic H2S release from anoxic zones also have to be considered.
The needs for an alternative physical, abiotic treatment technology that can replace the more sensitive biological treatment processes seem obvious. A development somehow similar to the automatization of the laborious and manual process of washing dishes using dishwasher machines.
Electrochemical water treatment
Electrochemical water disinfection is a process where electrons delivered by direct current react with ions and molecules in the water. The basic requirement for the process is a power supply and two electrodes, an anode and a cathode. Based on the electrode materials, current applied and water composition, various redox processes takes place at different rates. Different terms have been used to describe this type of water treatment process, including Forced Redox, Electrolytic disinfection, Electrochemical disinfection, Functional water, Electrochemical oxidation process (EOP), Electrochemical active water, Electrocoagulation, and Hydroxyl radical production.
Different reactions and processes take place at both electrodes during electrolysis of water. The most common application of EOP is for disinfection, based on electro generation of reactive oxygen species (ROS). The same technology can also be applied to oxidize complex, slowly degradable compounds and dissolved nutrient such as ammonium. Various studies of electrochemical advanced oxidation processes for water treatment of various water matrices have recently been published, including interesting aquaculture applications.
In our study, we tested EOP using micro flow cells (ElectroCell) in a bench scale setup using water from recirculating aquaculture systems (RAS). The two objectives were to test the potential of EOP to oxidize ammonium nitrogen and reduce the amount of dissolved N; and to investigate the disinfection capacity of EOP.
This study was a subtask of a work package about abiotic treatment of aquaculture discharge as part of a European innovation project, Bonus CleanAq funded by the EU and the Innovation Fund Denmark. We thank Ulla Sproegel, Brian Møller, Carine B. Jensen (DTU Aqua, Section for Aquaculture) for lab support.
Electrochemical TAN removal
To evaluate the electrochemical removal of total ammonia nitrogen (TAN), we tested different combinations of electrodes in a bench scale setup (see image below) with freshwater and brackish water of 8 ppt salinity. Water samples – either a mix of tap water and seawater, or RAS water mixed with seawater – were spiked with NH4Cl at a concentration equivalent to 30 ppm TAN and used in the closed setup. The setup included a volume of 1-L with a flow rate of 920 mL/min passing a micro flow cell with two electrodes with a contact area of 10 cm2 (0.001 m2), with an electric potential of 5 Volts and a current density of 22 mA/cm2.
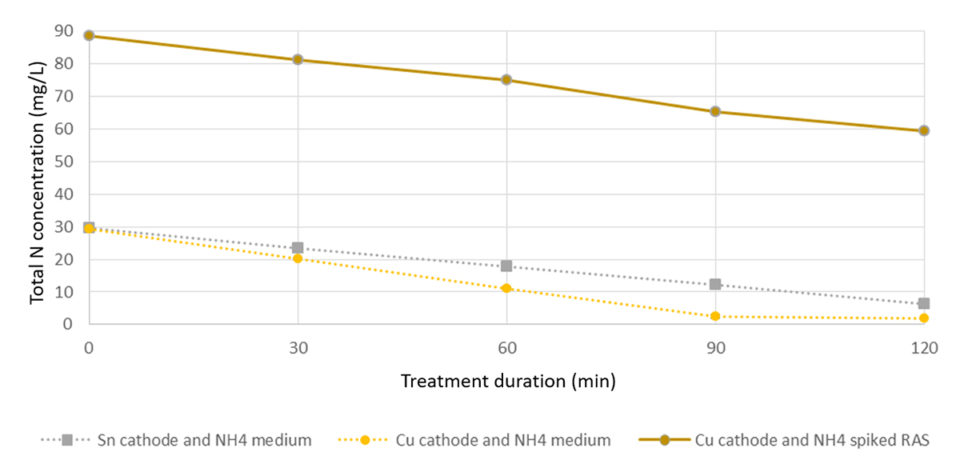
Over a period of two hours, up to 28 mg/L TAN was removed using a combination of copper cathode and boron-doped diamond (BDD) electrodes. Combinations of BDD- tin (Sn; anode and cathode, respectively) were also found to be effective (23 mg TAN/L removed), whereas BDD-BDD electrodes only showed limited TAN removal capacity.

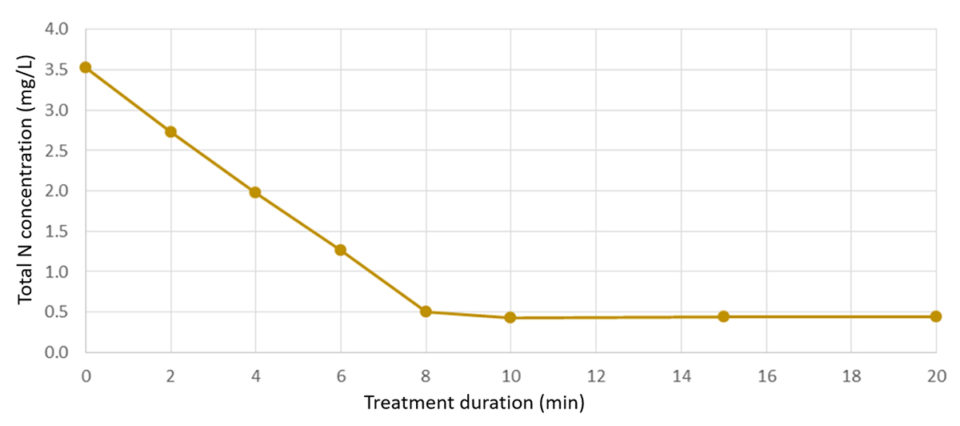
When extrapolated to larger scales, TAN removal rates up 0.45 kg TAN per day per square meter were determined for a copper-BDD electrode. Similar rates were found in brackish RAS with a lower TAN level (3.5 ppm) – where the same cell oxidized 70 percent of the added TAN within 7 minutes and lowered the TAN concentration to 0.5 ppm, which remained stable throughout a testing period of 20 min. The electrochemical surface specific TAN removal here corresponded to approx. 0.48 kg N per day per square meter.These finding were confirmed with measures of total dissolved N, which followed the same rate of degradation. The estimated electrochemical N-removal was found to be in the range of 40 KW/kg N.
A linear reduction of ammonium and total dissolved N was found at high and low TAN concentrations (Fig. 1). The copper electrode removed ammonium at a higher rate compared to the tin electrode. At lower TAN levels, copper-BDD combinations showed an immediate and constant removal of ammonium down to 0.5 mg N/L.
Electrochemical disinfection
The potential disinfection capacity of a flow-cell with combined BDD-BDD electrodes was tested in freshwater and in brackish RAS water. Furthermore, an ozonizer treatment device called “Doctor Chihiro” was also evaluated in batch scale experiments. The water samples originated from a pilot scale freshwater RAS and from a commercial saltwater RAS or mixtures hereof. The studies did not quantify the composition of radicals and oxidants formed during electrochemical oxidation but measured the total amount of oxidants (also referred to as total residual oxidants, TRO) as chlorine (Cl2) equivalent. Here, we found linear positive correlations between current density, salinity and exposure time on the formation of oxidants.
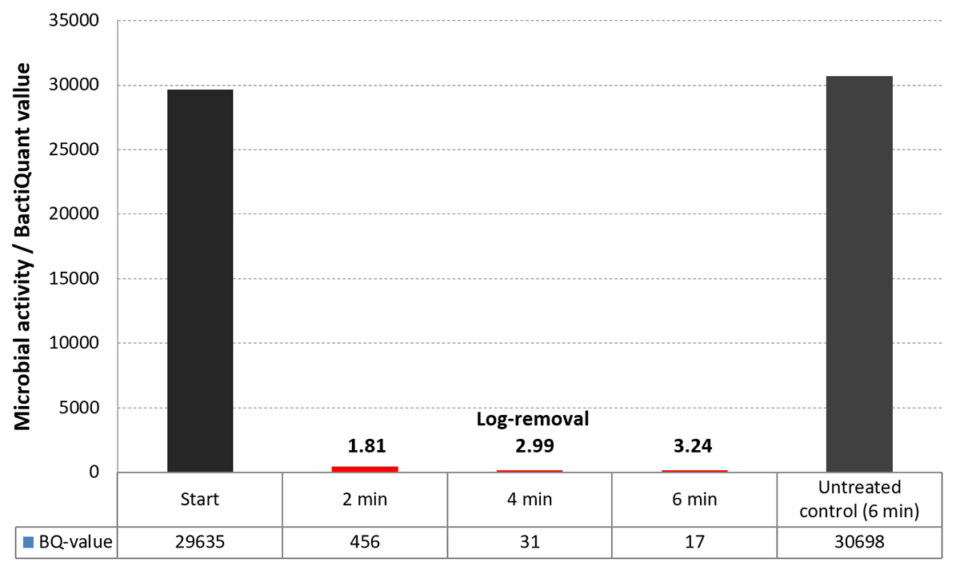
In addition, the electrochemical disinfection efficacies were evaluated by use of new microbial assays quantifying the bacterial activity in the water. The microbial activity in the water was tested on RAS water samples prior to, during and after electrochemical treatment. The degrees of bacterial inhibition were proportional to the oxidants produced at the batch experiments and showed that a 1,000-fold reduction (Log3) could be achieved within 5 minutes of electrochemical oxidation (Fig. 3).
Potential use in aquaculture
Summarizing our overall findings, it is possible to remove total ammonia nitrogen (TAN) by electrochemistry, and reactive oxygen species (ROS) were found to be formed in freshwater, and particularly in brackish water, with high antimicrobial effect. The study did not address quantification of the oxidants formed, so the toxic effects on fish and the safety issues need more attention before further upscale and potential implementation.
Provided these issues can be effectively addressed, electrochemical oxidation may be a future alternative treatment technique that can be considered, for example, during live fish transport where ammonia excretion and accumulation is critical and water volumes are low. Furthermore, the electrochemical oxidation process (EOP) can potentially reduce the start-up time required for (very) cold water RAS facilities, where biofilter maturation can require months before nitrification is established.
EOP can potentially be applied for disinfection of aquaculture systems. In situations where severe disinfection (sterilization) is required to eradicate pathogens or if cleaning between batches is needed (all-in all-out), the EOP can produce potent oxidants that can be circulated within the RAS and hence disinfect microorganisms in the water and on surfaces, including biofilms.
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Authors
-
Lars-Flemming Pedersen, Ph.D.
Corresponding author
Technical University of Denmark
DTU Aqua, Section for Aquaculture
The North Sea Research Centre
P.O. Box 101, DK-9850 Hirtshals, Denmark -
Anna Maria Røyset Lier, M.Sc.
University of Life Sciences
Faculty of Chemistry, Biotechnology and Food Science
Ås, Norway -
David Jonathan Jensen, M.Sc.
Technical University of Denmark
DTU Aqua, Section for Aquaculture
The North Sea Research Centre
P.O. Box 101, DK-9850 Hirtshals, Denmark
Tagged With
Related Posts

Innovation & Investment
Texas producer giving RAS shrimp production a charge
U.S. RAS shrimp producer NaturalShrimp uses electrocoagulation to remove and control bacteria, ammonia and other contaminants that can hinder production.

Health & Welfare
Electric company: Handheld CQR aims to crack fish-health code
U.S. company’s certified quality reader utilizes electrodes to create signals that provide valuable information about fish health in just seconds.

Innovation & Investment
American Unagi brings eel farming back ‘home’
Sara Rademaker launched American Unagi to shift eel farming to American soil, where the eels are from. Why? Because of the novelty, and because she saw an opportunity to do things better.

Intelligence
RAS believers making an early stand on American soil
RAS is an emerging sector with two producers leading the U.S. pack, responding to high demand for Atlantic salmon and for local, low-impact food.


