pH of stripped eggs can predict catfish hatching success

Egg quality in fish aquaculture can be defined as the ability of eggs to be fertilized and to subsequently develop into normal embryos. Variation in the egg quality of cultured fish is an important limiting factor for fry production and a major obstacle for large-scale aquaculture production.
In fish, eggs are the final product of oocyte growth and development, a process that can take up to a year. All the components of an egg, genetic and nutritional factors determine the quality, and must be incorporated at the oocyte stage within the ovary. The eggs of fish, which measure up to 1 mm in diameter, are more than 23,000 times larger by volume than human eggs. Developing oocytes autonomously make most of the needed components of the machinery for DNA, protein synthesis and mRNA after fertilization.
Although current knowledge is limited, understanding of the mechanisms involved in oocyte growth and development is essential to our understanding of the factors affecting egg quality. Known factors that affect egg quality include genetic effects, endocrine effects, husbandry practices, broodfish diets and physiochemical conditions during spawning and incubation. Other factors, such as maternal and paternal genetic components, environmental factors and the roles of maternal RNA during oogenesis and embryogenesis, may also be involved in the making of a “good quality” egg.
In teleosts, six phases of oocyte growth are broadly classified from ogenesis to maturation and ovulation. The production of a good-quality egg relies upon correct progression through each of these phases in a coordinated “assembly” controlled by endocrine and intraovarian factors.
In aquaculture, the need for a precise estimation of egg quality is extremely important to clarify, for example, if low survival during early larval rearing is due to the initial viability of larvae or the quality of the eggs. Hatchery production can be optimized by starting with the production of high-quality eggs, which would result in higher hatching and survival in the hatchery and subsequent culture environments. Identification of a method to reliably discriminate between low- and high-quality egg batches is needed.
Past studies
Egg and sperm constitute gamete quality. Fertilization success is probably one of the earliest estimators that can be recorded accurately to estimate egg quality. It is also the most integrative estimator of sperm quality.
Research by Niall Bromage and co-workers in 1994, as well as 1997 research by Suzanne Brooks and team concluded that the symmetry of cell division can be considered an indicator of normal hatching and larval development in fish. They evaluated several morphological and biochemical parameters to predict egg quality, such as female weight, oocyte weight, adenosine triphosphate concentration, ovarian fluid pH, osmolarity, protein concentration, survival at different developmental stages, proportion of eggs fertilized, hatch of eyed eggs and swim-up fry.
Present research
Natural spawning of channel catfish in ponds answers the fry and fingerling needs of the farm-raised channel catfish industry in the United States. Catfish spawns collected from ponds are generally of good egg quality.
In order to reduce production costs and increase profitability in catfish farming, an increasing number of farmers are raising hybrid catfish in production ponds. Hybrid catfish have higher growth rates, survival and processing yield than commonly raised channel catfish. However, hybrid catfish fry production is a hormone-based synchronization protocol consisting of collecting eggs from strippable, induced channel catfish females, fertilizing them with blue catfish sperm and incubating the fertilized eggs to hatch fry.
Stripping eggs from hormone-induced channel catfish females often results in lower and varying degrees of egg quality. This may be a consequence of the hormone administration, varying stages of maturation or mechanical damage from the stripping process. No effective predictive marker exists in strip-spawned channel catfish eggs, even though non-viable stripped eggs can be occasionally identified by their whitish color and less-glossy appearance.
Preliminary research has identified that the pH of the ovarian fluid of stripped channel catfish eggs could provide an empirical measure of egg quality to predict subsequent hatch rates. At the United States Department of Agriculture’s Agricultural Research Service Catfish Genetics Research Unit in Stoneville, Mississippi, USA, the author evaluated the feasibility of using the pH values of stripped unfertilized eggs to predict subsequent fertilization and hatch rates.
Experimental design
Four-year old mature channel catfish females were hand selected and induced to spawn with luteinizing hormone-releasing hormone. The stripped eggs of an individual fish were weighed in greased pie pans. The in vitro pH of the ovarian fluid and egg mass were measured with an electrode using a commercial pH meter.
In the study, 71 females were strip spawned, and two samples of 400 eggs from each female were weighed, fertilized with blue catfish sperm, activated with hatchery water and water hardened to obtain an adhesive spawn. Individual egg spawns were held in mesh cups in an 80-L aquarium.
Table 1 shows the mean fertilization and hatch rates of hybrid catfish embryos produced from stripped unfertilized eggs of channel catfish. A positive relationship was established between hatch rate and the ovarian fluid pH of the stripped eggs of the catfish. This relationship suggested the ovarian fluid pH of stripped channel catfish eggs prior to fertilization can be predictive of the hatching success of hybrid catfish eggs (Figure 1).
Chatakondi, Mean fertilization and hatch rates, Table 1
| pH of Stripped Eggs | Eggs Spawn Tested | Fertilization (% | Hatch (%) |
|---|
pH of Stripped Eggs | Eggs Spawn Tested | Fertilization (% | Hatch (%) |
|---|---|---|---|
| Low pH (pH < 7.0) | 14 | 76.7 ± 2.6a | 8.2 ± 0.8a |
| Medium pH (pH 7.0-7.4) | 25 | 87.3 ± 3.8b | 24.6 ± 1.1b |
| High pH (pH > 7.4) | 32 | 90.0 ± 1.1b | 41.9 ± 0.5c |
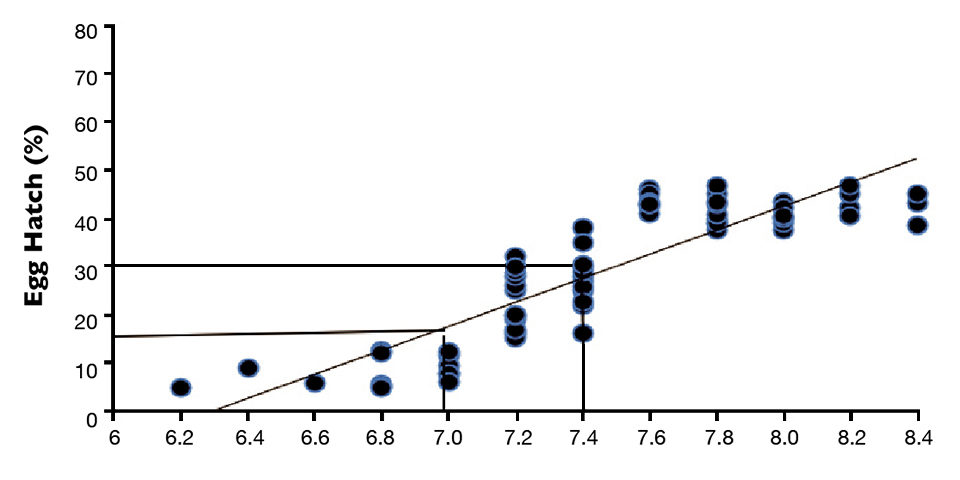
The hatching percentage criteria were used to categorize the pH of stripped eggs. A pH value below 7.0 identified “low pH’’ eggs, pH of 7.0 to 7.4 indicated “medium pH” eggs, and eggs with a higher pH were considered “high pH.”
Perspectives
Production of hybrid catfish is an expensive and labor-intensive process because it involves manipulation of individual channel catfish and blue catfish. To minimize costs and labor, it is essential to optimize every aspect of reproduction and early life-stage development in the hatchery.
Overall, egg viability is the most important quantitative measure of hatchery efficiency. Egg quality can be assessed by visual inspection of eggs in association with simple measurements of the pH of stripped eggs. Hatchery personnel can use these techniques to quickly identify low-quality eggs and separate or discard them prior to fertilization.
The removal of low-quality eggs reduces the use of hatchery resources and the spread of fungal disease to healthy eggs, and improves hatchery efficiency. Measures of the pH of stripped eggs can also be used in research as an objective measure of egg quality when studying broodfish management practices.
(Editor’s Note: This article was originally published in the November/December 2012 print edition of the Global Aquaculture Advocate.
Author
-

Nagaraj G. Chatakondi, Ph.D.
USDA Agricultural Research Service
Catfish Genetics Research Unit
Thad Cochran National Warmwater Aquaculture Center
P. O. Box 38
Stoneville, Mississippi 38776
Tagged With
Related Posts

Innovation & Investment
Assessing coloration in channel catfish fillets
Because consumers look at color to gauge quality of catfish fillets, the authors developed a digital photography measurement method to assess yellowness.

Health & Welfare
Biofloc technology reduces common off-flavors in channel catfish
In studies that used biofloc systems to culture channel catfish, culture tanks were susceptible to episodes of geosmin and 2-methylisoborneol and subsequent bioaccumulation of off-flavors in catfish flesh.

Responsibility
A look at integrated multi-trophic aquaculture
In integrated multi-trophic aquaculture, farmers combine the cultivation of fed species such as finfish or shrimp with extractive seaweeds, aquatic plants and shellfish and other invertebrates that recapture organic and inorganic particulate nutrients for their growth.

Health & Welfare
Advances in intensive copepod production technology
Research at the Oceanic Institute has been successful in overcoming bottlenecks associated with rearing small-mouthed fish larvae by finding a suitable first feed. Early work on the calanoid copepod Parvocalanus crassirostris focused on parameters necessary for successful maintenance of stock cultures.

