Results show a near 56% efficacy rate, but larger-scale trials are needed

Atlantic salmon (Salmo salar) is reportedly the most economically important species in aquaculture globally. However, increased production has resulted in a larger number and stronger impact of diseases, with parasitic salmon lice becoming one of the most important in recent years in all the major salmon-producing countries.
Two lice species are of primary concern for salmon farming: Lepeophtheirus salmonis in the Northern Hemisphere and Caligus rogercresseyi in the Southern Hemisphere. In our study, we focused on the former species, L. salmonis, which predominates in the North Atlantic and causes year-round infestations of Atlantic salmon reared in marine cages, with associated consequences for fish health in both farmed and wild salmonids as well as for aquaculture economics and sustainability.
Sea lice parasitize salmon during the marine phase of the life cycle, in both wild and farmed salmon, by attaching to their skin, often close to gills and fins; feeding on the mucus, epithelial tissues and blood; reproducing on the host; and releasing the eggs into the seawater. If left untreated, this can lead to impaired growth, osmoregulatory stress and open wounds, which can facilitate the entry of other pathogens. The impaired growth and secondary infections cause significant negative animal welfare and economic impact. Moreover, relative to other salmonids, Atlantic salmon have limited ability to resist infection by L. salmonis and are therefore highly susceptible to the parasite.
Vaccination against salmon lice could be an important alternative to control this parasite, because vaccines have greatly contributed to reducing the use of drugs (especially antibiotics) against several fish diseases. In our study, we used a previously produced, candidate vaccine based on a protein (ribosomal protein P0) – which had shown potential in a previous study we conducted – to validate at the laboratory scale.
This article – adapted and summarized from the original – reports on a study that investigated if this candidate vaccine could provide protection to Atlantic salmon in terms of reduced lice count or reduced fecundity, or both.
Study setup
The experiment was performed at the Aquaculture Research Station (Tromsø, Norway). Atlantic salmon (AquaGen standard, average weight 40 ± 6 grams) were maintained at a density of 10 kg per cubic meter in circular, 500-liter tanks with filtered circulating water for two weeks at an ambient temperature of approximately 10 degrees-C with 24 hours of light (summer stimuli) for acclimation. Fish were fed with a commercial pellet diet (Nutra Olympic, Skretting).
Three 500-liter tanks were stocked with 120 fish each, one tank assigned to each of the three experimental groups: procedural control (Group 1), injected vaccine (Group 2), and injected vaccine + bath immunization (Group 3). Each tank was supplied with continuous circulating water flow throughout the experimental period and oxygen level and temperature were recorded daily.
For vaccine formulation, recombinant antigen protein [an antigen is a molecule that elicits an immune response (antibody production) in the host; recombinant proteins are engineered forms of native proteins that are produced using molecular biology techniques] or phosphate-buffered saline, PBS [a water-based salt, buffer solution commonly used in biological research that helps maintain a constant pH] control were prepared. The immunization and challenge schedules are outlined in Fig. 1. The fish were starved for one day before vaccination and anaesthetized.
The first immunization was performed as follows: each fish in the control group (Group 1) received 0.05 mL of PBS emulsified in adjuvant [ingredient used in some vaccines to help produce a stronger immune response] by intraperitoneal (ip) injection [injection of a substance into the peritoneum or body cavity]. The second group (Group 2) received an ip injection at a dose of 1 μg per gram of body weight (gbw) of the recombinant antigen protein emulsified in adjuvant. The third group (Group 3), received an ip injection at 1 μg per gbw of the recombinant antigen protein emulsified in adjuvant plus bath immunized with recombinant antigen protein as inclusion bodies (200 μg/L) for 1 hour (120 fish in 200-liter aerated static bath), immediately after ip injection.
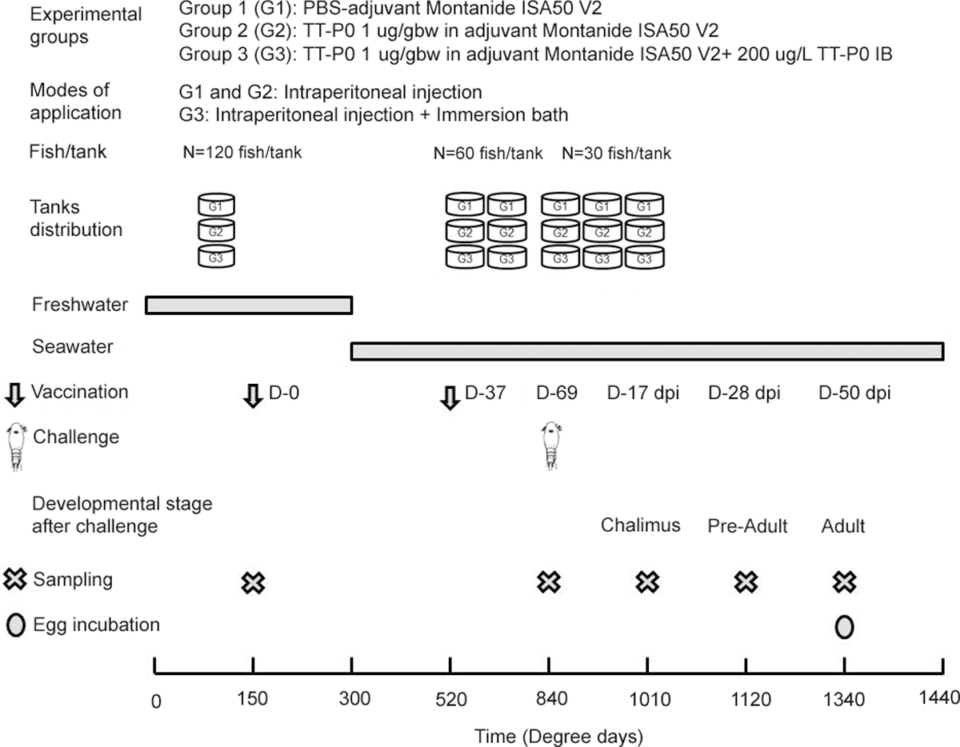
For additional, detailed information on the experimental design; fish husbandry; antigen purification; vaccine formulation and fish immunization; lice challenge, sampling and counting; vaccine efficacy; gene expression and other relevant components of this study; and statistical analyses, refer to the original publication.
Results and discussion
The importance of Atlantic salmon in aquaculture and its susceptibility to infestation with L. salmonis has stimulated scientists to investigate efficient, non-medicinal, cost-effective and eco-friendly measures to control sea lice loads through possible vaccine development. In our study, we used a vaccine previously tested for better antibody response [the antigen-antibody reaction is a specific chemical interaction between antibodies produced by white blood cells and antigens (a molecule or molecular structure, such as may be present at the outside of a pathogen, that can be bound to by an antigen-specific antibody) during an immune reaction].
Based on our sampling results, initially an overall average of about 23 attached lice at the chalimus (pre-adult) stage were recorded from each fish sampled at 17 days post-infection, dpi. However, by the end of the experiment, these numbers had declined to about five adult lice per fish.
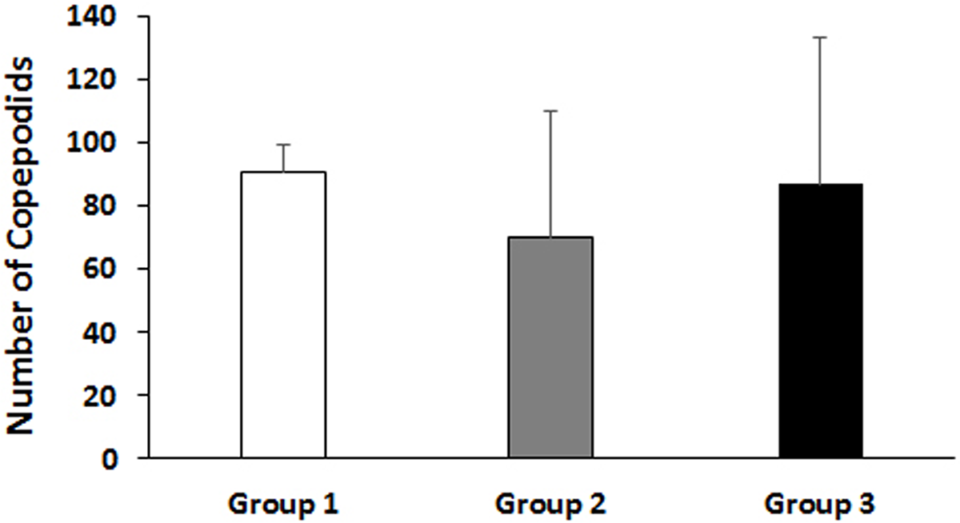
The total number of lice attached did not vary statistically between the immunized or control groups of fish, although there was a tendency of reduction at the adult lice stage in the ip vaccinated group 2. However, we documented a significant impact on gravid female lice count and its reproductive efficacy with delayed hatching and a reduced trend of copepodid (free-swimming larval stage of sea lice) counts in the first, F1, generation in group 2 compared to the control group. This showed that the major effect of the vaccine was apparent in the adult female lice and its fecundity.
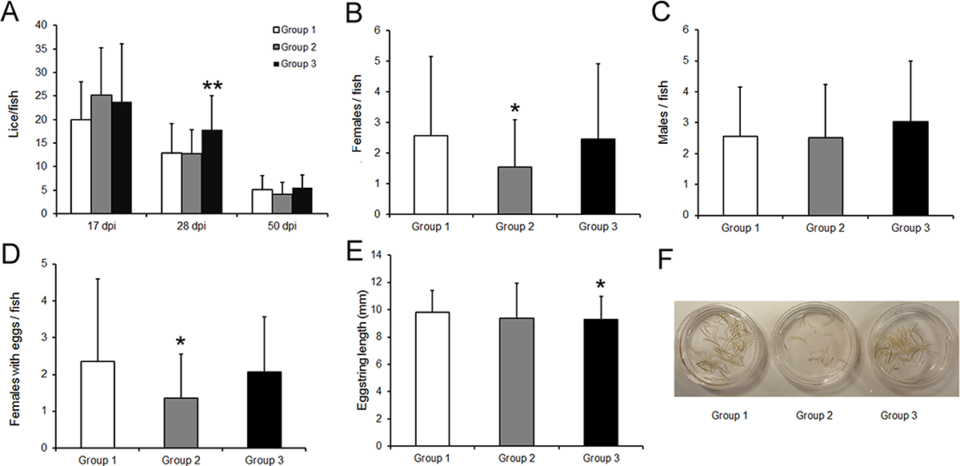
Other studies have reported similar results using sea lice whole extract or lice protein involved in midgut function and blood digestion, as a vaccine in Atlantic salmon, resulting in fewer oviparous (egg laying) female lice and lower fecundity. Based on our results, it is expected that a reduction in parasite fecundity due to vaccination will have an exponential reduction effect on the overall lice population, and thus on the lice load on the host at later generations, and consequently will permit a reduction in chemical, or drug free treatments to control lice.
We applied a formula to our experiment for estimating vaccine efficacy in order to evaluate the impact of the vaccine candidate on the lice fecundity as well as on the hatching and survival of F1 copepodids. Based on this formula, vaccinated Group 2 had an overall vaccine efficacy of 56 percent, whereas Group 3 had 25 percent efficiay, suggesting the utility of the vaccine candidate through the ip method only. However, further in-depth work has to be done.
Moreover, lice count as a proxy for resistance has been questioned by some researchers, because individual lice counts vary between trials and certain immune genes are affected negatively by increasing number of lice. Therefore, large number of experimental animals and experimental tanks must be used in these types of immunization and challenge trials, and treatment efficacy parameters other than lice count should be considered.
Bar graph showing total number of copepodids 10 days post incubation of egg strings. Fifty egg strings (sampled from the first reproductive event at 50 dpi) from each experimental group were randomly distributed and incubated in five parallel aerated flow-through incubators (containing 500 mL filtered seawater/incubator at ~10 degrees-C) having 10 egg strings in each incubator. The bar shows the mean value + SD in 5 replicate incubators for each experimental group. Group details: Group 1 is control group; Group 2 received an ip injection of adjuvant emulsified vaccine antigen; Group 3 received an ip injection of adjuvant emulsified vaccine antigen + bath immunization.
Our results showed that the ip injection of the candidate vaccine plus an immersion bath with inclusion bodies received by Group 3 was less effective, although some immune parameters were improved. The differences we observed in the regulation of inflammation could explain the differences found in the results between different vaccination methods. Further studies targeting more immunological markers could clarify the mechanisms responsible for the differences between the two groups.
Perspectives
Results of our study provide new insight into the potential of the candidate vaccine in reducing salmon lice load and its effect on host-parasite interaction with minimal side-effects. The calculated vaccine efficacy of 56 percent in the ip injected vaccine group suggests a larger impact on F1 parasite generation by reduced re-infection loads via fewer females and decreased fecundity. In addition, the results revealed the priming of immune response post vaccination and pre-challenge, leading to simultaneous involvement of both systemic and local immunity during the salmon lice interaction for vaccinated fish, at the mobile lice stages.
These findings provide valuable support for the effectiveness of the P0 antigen as a vaccine candidate against L. salmonis. However, long-term challenge trials with larger number of test fish per tank and studies of re-infection post vaccination are necessary to fully understand and explore the protection potential of this candidate vaccine and the underlying molecular mechanism of protection at the gene level.
Another aspect to consider is that under experimental challenge conditions, the infestation load is usually very high – 35 copepodids per fish in our study – much higher when compared to the natural conditions. Therefore, carrying out a challenge experiment considering these conditions will be the next step for additional evaluation of the vaccine efficacy in controlling salmon lice infestation.
References available in original publication.
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Authors
-
Jaya Kumari Swain, Ph.D.
Corresponding author
Fish Immunology and Vaccinology Research Group
Norwegian College of Fishery Science
UiT – The Arctic University of Norway
Tromsø, Norway -
Yamila Carpio, Ph.D.
Animal Biotechnology Division
Center for Genetic Engineering and Biotechnology
Havana, Cuba -
Lill-Heidi Johansen, M.Sc.
Nofima – The Food Research Institute
Tromsø, Norway -
Janet Velazquez
Ph.D. student
Animal Biotechnology Division
Center for Genetic Engineering and Biotechnology
Havana, Cuba -
Liz Hernandez, M.S.
Animal Biotechnology Division
Center for Genetic Engineering and Biotechnology
Havana, Cuba -
Yeny Leal
Ph.D. student
Animal Biotechnology Division
Center for Genetic Engineering and Biotechnology
Havana, Cuba -
Ajey Kumar, Ph.D.
Symbiosis Centre for Information Technology
Symbiosis International (Deemed University)
Pune, Maharashtra, India -
Mario Pablo Estrada, Ph.D.
Animal Biotechnology Division
Center for Genetic Engineering and Biotechnology
Havana, Cuba
Tagged With
Related Posts

Health & Welfare
Development of DNA vaccine for piscine mycobacteriosis
Wild and cultured fish around the world are commonly affected by piscine mycobacteriosis, infections of which often manifest as acute or chronic diseases.

Health & Welfare
Emerging epitheliocystis disease in Mediterranean sparids caused by novel bacteria
Epitheliocystis is an emerging infection among farmed gilthead seabream and is also lethal in mesocosm cultures of sharpsnout seabream larvae. Two studies of this disease at sites in Greece and Crete have characterized the gill and skin cysts in more detail.

Innovation & Investment
Global Aquaculture Innovation Award finalist: VakSea
An orally administered vaccine product holds great promise for aquaculture, and Maryland-based startup VakSea is on the cusp of providing one. Sensing a major opportunity, the company’s focus has pivoted to shrimp.

Innovation & Investment
Scottish firm honing bacteriophages into aquaculture-disease assassins
Scottish biotech firm Fixed Phage aims to bottle the powers of bacteriophages to deploy these “bacteria killers” on some of the world’s most destructive aquaculture diseases.


