Results suggest TiLV can be transmitted vertically to reproductive organs, fertilized eggs

Tilapia Lake Virus (TiLV) is a newly discovered virus of tilapines (a tribe within the fish family Cichlidae) and its infections have been documented in either farmed or wild tilapines populations in 14 countries around the world, including Asia, Africa and South America. Natural outbreaks of TiLV reportedly resulted in 20 to 90 percent mortality, and experimental infection using cultivated virus caused 66 to 90 percent mortality in tilapia juveniles. Current literature shows that TiLV seems to be most important in tilapia fry, fingerlings and juveniles although viral infections were also evidenced in sub-adult and adult fish. Additionally, subclinical infections with minor mortality impact have also been reported.
Horizontal transmission (transmission of infections between members of the same species that are not in a parent-progeny relationship) of the virus has been confirmed through cohabitation of infected fish with clinically healthy fish, which resulted in 80 percent mortality, confirming waterborne transmission. Experimental challenge of nine non-tilapia fish species with TiLV revealed that giant gourami (Osphronemus goramy; commercially important in aquaculture) is an additional susceptible species and cohabitation between tilapia and giant gourami caused cross-species transmission.
Sizing up TiLV and its potential impact on tilapia production
A preliminary investigation we conducted indicated that there was a proportion of apparently healthy tilapia broodstock that tested positive for TiLV. In tilapia hatcheries, detection of TiLV in fertilized eggs and very early developmental stages of tilapia, e.g., yolk-sac stage, fry and fingerlings suggest possible vertical transmission of this virus. However, it is still unclear whether TiLV is able to transmit from infected broodstock to their reproductive organs and progeny.
This article – adapted and summarized from the original publication [Dong HT et al. 2020. Experimental infection reveals transmission of tilapia lake virus (TiLV) from tilapia broodstock to their reproductive organs and fertilized eggs] – reports on research to investigate whether experimentally infected broodstock can pass the virus to their reproductive organs, eggs and sperm as well as in vitro fertilized eggs.
This work was undertaken as part of, and funded by, the CGIAR Research Program on Fish Agri-Food Systems (FISH) led by WorldFish. The program is supported by contributors to the CGIAR Trust Fund. The authors thank S. Taengphu, W. Meemetta, P. Meenium, and M. Bunnontae for their skilled technical assistance.
Study setup
Tilapia lake virus strain NV18R from diseased red tilapia (Oreochromis sp.) were cultivated and isolated, and purified viral stock was then confirmed by TiLV-specific PCR, and an injection dose of 105.5 tissue culture infective dose (TCID50) per fish established.
Five pairs of fully mature Nile tilapia broodstock (300 to 400 grams) were obtained from a commercial tilapia hatchery with no history of TiLV infection, and each pair of broodstock cultured in 50-liter fiberglass, aerated tanks. The fish were fed twice daily with commercial tilapia feed pellets containing 28 percent crude protein at 5 percent body weight.
At day 6 post-infection, there was still no sign of any abnormal symptoms. The ovulated eggs and semen from each broodstock were collected and mixed with the semen collected from the male in the same family. The remaining unfertilized eggs were also kept for further PCR and viral culture experiments. For each diagnostic test, pools of 10 unfertilized or fertilized eggs at each time course were used. Blood samples were also collected from the fish, which were then euthanized and dissected to collect livers and reproductive organ collection. These tissues were preserved for PCR, histology, and re-isolation onto cell cultures.
For detailed information on virus preparation; fish infection, in vitro fertilization, and sample collection; semi-nested RT-PCR detection of TiLV; histology and in situ hybridization; and viral re-isolation using fish cell line, refer to the original publication.
Results and discussion
In this study, tissues from three families of the experimental TiLV-infected tilapia broodstock as well as their in vitro fertilized eggs were subjected to TiLV detection by Polymerase Chain Reaction (PCR; a widely used, molecular biology method that rapidly makes millions to billions of copies of a specific DNA sample for detailed study). Liver, blood, ovary and unfertilized eggs were collected from female broodstock and liver, blood and testis from male fish were used. Artificially fertilized eggs were collected at 3, 12 and 64 hours post-fertilization. Corresponding specimens from the two control families were similarly processed.
The results demonstrated a systemic infection with TiLV in the experimentally challenged fish. All tested samples from the challenged families – including the liver, blood, reproductive organs of both male and female broodstock, and the unfertilized eggs – tested positive for TiLV by PCR. The fertilized eggs at 3, 12 and 64 hours post-fertilization collected from three infected families (1 to 3) were also positive, except for samples collected at 3 and 64 hours from family 1. Samples from two control families (4 and 5) were all negative for TiLV. Representative PCR testing results from an infected and non-infected control family are shown in Fig. 1.
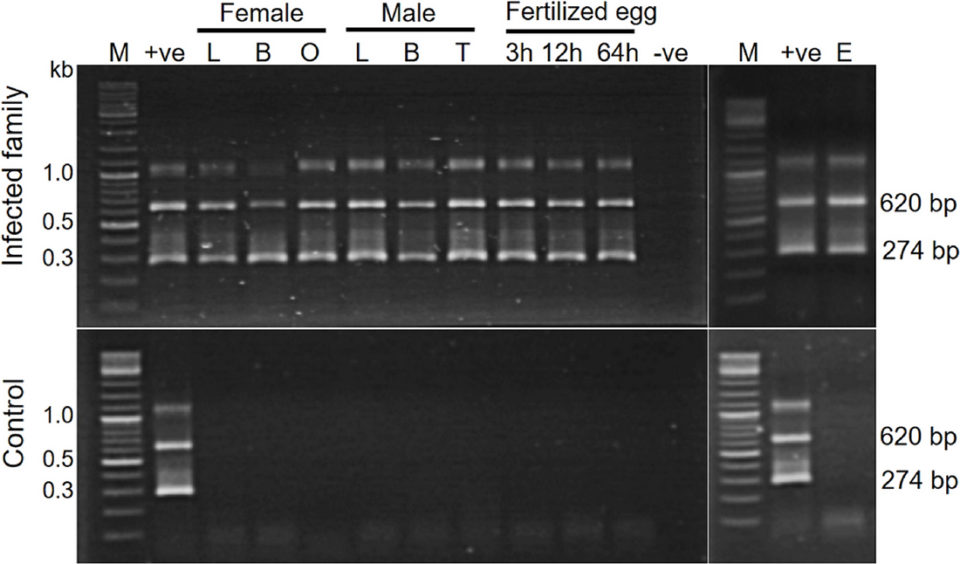
Understanding disease pathogenesis and transmission route of TiLV are important scientific bases for further studies on effective prevention approaches. In our study, despite the fact that the virus was injected intramuscularly into broodstock, the virus was later detected in the blood, liver and reproductive organs, which was confirmed by PCR, In Situ Hybridization (ISH; a type of hybridization that uses a labeled complementary DNA, RNA or modified nucleic acids strand [i.e., probe] to localize a specific DNA or RNA sequence in a portion or section of tissue) and cell culture. Large areas surrounding blood vessels of the tissues assayed (liver and testis) strongly reacted with TiLV-specific probe by ISH. As far as we know, this has not yet been reported for TiLV infection. These findings suggest that TiLV is systemic in nature and spreads to other organs most probably through the circulatory system.
To the best of our knowledge, this is the first laboratory-controlled study to prove that experimentally infected broodstock can pass the TiLV virus to their reproductive organs. We observed that – based on the comparative frequencies of strong, widespread of ISH reactive signals in the reproductive organs – viral infection in the ovary seems to be more severe than in the testis.
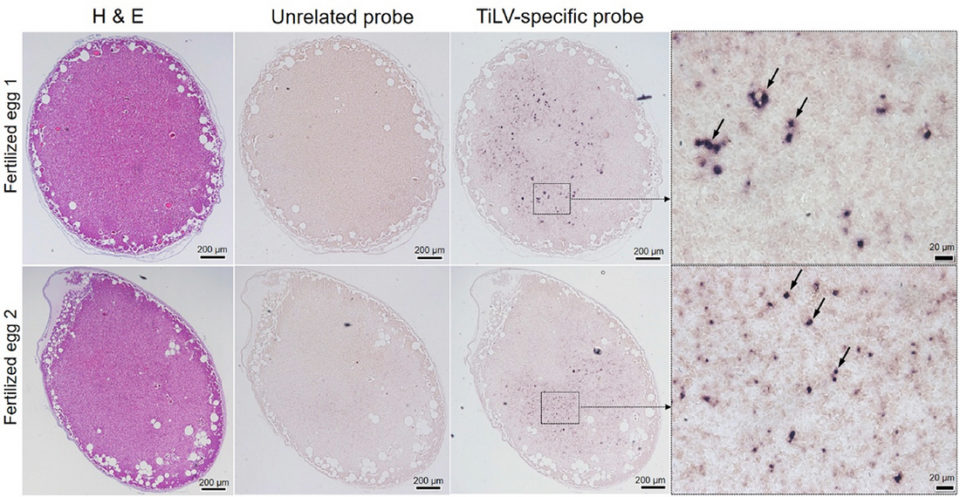
Therefore, despite the fact that in our experimental infection challenge, TiLV was able to transmit vertically to fertilized eggs, it remains unknown whether those infected fertilized eggs would be able to develop to fry and fingerlings and whether the virus would still be infectious through their development stages. These questions should be further investigated.
Perspectives
Results of our study suggests that TiLV caused systemic infection in tilapia broodstock and that the virus is capable to circulate into the reproductive organs of both male and female via the blood circulatory system. The infected female fish produced infected eggs, when fertilized with sperms from infected male broodstock resulted in infected fertilized eggs. Therefore, to produce TiLV-free seedstock, we highly recommend using TiLV-free broodstock.
References available from the original publication.
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Authors
-
Ha Thanh Dong, Ph.D.
Corresponding author
Faculty of Science and Technology
Suan Sunandha Rajabhat University
Bangkok, Thailand[104,116,46,99,97,46,117,114,115,115,64,111,100,46,104,110,97,104,116,97,104]
-
Saengchan Senapin, Ph.D.
Fish Health Platform
Center of Excellence for Shrimp Molecular Biology and Biotechnology (Centex Shrimp)
Faculty of Science, Mahidol University
Bangkok, Thailand -
Warachin Gangnonngiw, Ph.D.
Fish Health Platform
Center of Excellence for Shrimp Molecular Biology and Biotechnology (Centex Shrimp)
Faculty of Science, Mahidol University
Bangkok, Thailand -
Vuong Viet Nguyen, M.S.
Fish Infectious Diseases Research Unit (FID RU)
Department of Veterinary Microbiology
Faculty of Veterinary Science, Chulalongkorn University
Bangkok, Thailand -
Channarong Rodkhum, D.V.M, Ph.D.
Fish Infectious Diseases Research Unit (FID RU)
Department of Veterinary Microbiology
Faculty of Veterinary Science, Chulalongkorn University
Bangkok, Thailand -
Partho Pratim Debnath, M.S.
WorldFish
Dhaka, Bangladesh -
Jerome Delamare-Deboutteville, Ph.D.
WorldFish
Penang, Malaysia -
Chadag Vishnumurthy Mohan, Ph.D.
WorldFish
Penang, Malaysia
Tagged With
Related Posts

Health & Welfare
Sizing up TiLV and its potential impact on tilapia production
An international research effort has commenced to find a solution for Tilapia Lake Virus (TiLV), a contagion causing high rates of mortality in farmed and wild tilapia stocks in Israel, Colombia, Ecuador, Egypt and Thailand.

Health & Welfare
Hyperthermia can boost innate immune system in juvenile fish
Hyperthermia, or heat shock treatment, is an unconventional approach to pathogen control in fish aquaculture that appears to have potential to improve disease resistance in fish like tilapia and barramundi.

Health & Welfare
Evaluating effects of organic acids in Nile tilapia feed
A study tested the effects of a commercial product that includes three organic acids incorporated into a commercial feed for Nile tilapia fingerlings.

Intelligence
What will it take to make tilapia great again?
Once a darling of the sustainable seafood crowd for its vegetarian diet and potential to feed the world’s growing population, mild-mannered tilapia now has an image problem that may be causing a dip in consumption levels.


