Estimating nutritional requirements requires novel approaches
Production of marine fish juveniles in commercial hatcheries still generally depends on diets of live prey like rotifers and artemia. The substitution of compound diets for live prey, however, can lower production costs and sustain constant production of juveniles.
Weaning to compound diets in marine fish hatcheries is typically done after some weeks of life, while freshwater fish species can be fed compound diets as soon as their mouths open. During the last decade, the preweaning period for marine fish larvae has been greatly reduced, thanks to results obtained by feeding marine fish larvae compound diets from mouth opening.
Formulation
The formulation of compound diets for fish larvae is difficult, because estimating their nutritional requirements cannot be conducted using traditional nutritional approaches. Data from juvenile fish are of limited use when studying larval-stage requirements, because digestion and absorption mechanisms, and nutritional requirements change during larval development.
Digestion
The digestive tracts of fish larvae are not developed at hatching, and some have hypothesized a deficiency in digestive enzymes in very young larvae to explain unsuccessful feeding on compound diets. But enzymatic assays conducted after larval dissection in all segments involved in digestion (Fig. 1) have shown that fish larvae do not lack digestive enzymes. The specific enzymatic activity, expressed as the activity of the enzyme related to protein concentration of the larvae, demonstrates the digestive capacity of young larvae is very high, as related to their weight.
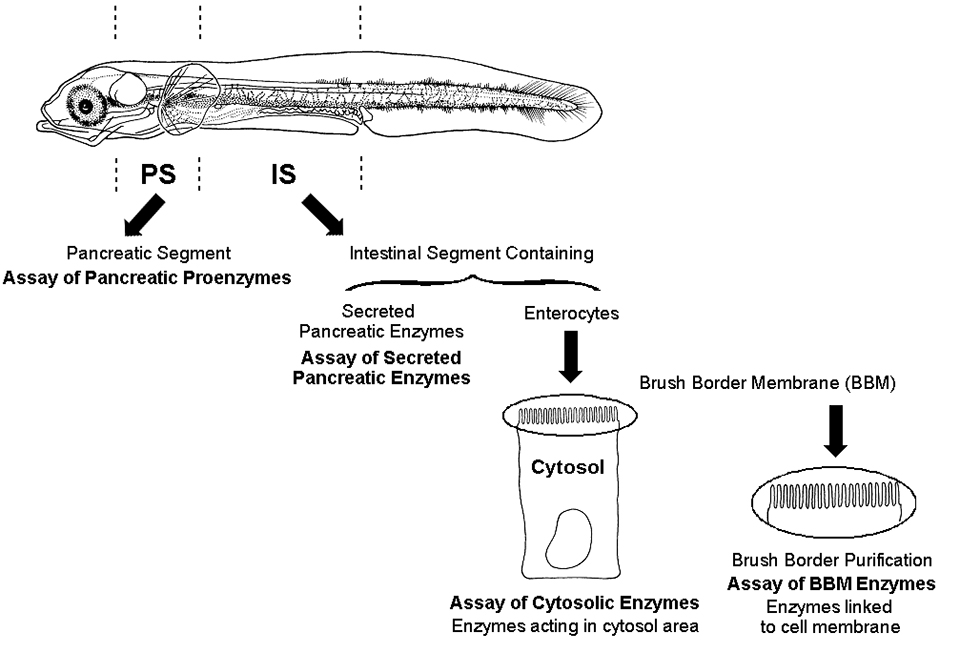
The onset of digestive functions, associated to morphological transformations, follows a sequential chronology in developing fish like that in developing mammals. Quantification of mRNA coding for several digestive enzymes showed that the pattern of digestive enzyme activity during larval development is genetically programmed.
Diet affects enzyme activity
Enzyme activity can be modulated by diet composition. For example, the activity of amylase, an enzyme that hydrolyzes glucides, is modulated by the level of starch in the diet fed to larvae. This modification in activity of pancreatic enzymes in response to diet changes is efficient from the earliest stages.
Cytosolic digestion
Fish larvae also show particularity in the intestinal phase of digestion. Some intestinal enzymes, such as peptidases, are located in cytosol of enterocytes (intestinal cells). Fish larvae show high activity of these enzymes, which hydrolyze short dipeptides and tripeptides.
These cytosolic enzymes decrease with age, whereas activity of enzymes located on brush border membranes increase abruptly around the third week of life in several species, including sea bass, sole, and red drum. The decrease in cytosolic enzymes and concurrent increase in enzymes of brush border membranes characterize normal maturation of the enterocytes in developing animals. Brush border membrane digestion represents an adult mode of digestion.
Nutritional requirements
Changes in larval digestive structures during larval development suggest differences in the nutritional requirements of larvae and juveniles. Indeed, a diet that sustains good growth in juveniles induces poor results in larval growth and survival. Recent studies have revealed specificities in larvae nutritional requirements.
Lipids
Growth and survival were directly related to dietary lipid content in sea bass larvae fed a microparticulated diet from mouth opening. Best results were obtained with the diet containing 25 to 30 percent lipid. Dietary lipid level determines the energy level, but lipid nature is also important.
Phospholipids are essential, and larval requirements are higher than those of juveniles. Both vegetable (soybean) and marine source (fish roe) phospholipids are adequate sources. Dietary phospholipid levels affect not only growth and survival in fish larvae, but skeletal formation, as well. Phosphatidylinositol, a phospholipid component, has been found essential for harmonious skeletal formation.
Highly unsaturated fatty acids
Lipid fraction must include highly unsaturated fatty acids, and their optimal level in marine fish larvae diets is 1.5-3.0 percent of dry matter. Docosahexaenoic acid (DHA) is more efficient than eicosapentaenoic acid for improving growth, survival, pigmentation, and tolerance to salinity stress or reduced dissolved oxygen. DHA is also involved in the neural and visual development of larvae.
Protein sources
The first compound diets formulated for fish larvae included several protein sources, so that any nutrient deficiency in a protein source could be compensated for by another one. Before moving toward formula simplification, several compound diets that included meals from fish, squid, shrimp, krill, and crab; gluten; hen eggs; and yeast were formulated and tried. Protein sources were selected following their amino acid profiles, and fishmeal or squid meal were incorporated in microdiets as the only protein source.
Protein levels
Optimum protein levels are higher in larvae and juveniles than in adults of the same species. This difference is attributed to the high growth rate and utilization of protein as energy in larvae. The best larval growth was observed with 50 percent protein, and a slightly slower growth was induced by 60 percent protein.
Amino acids and peptides
The molecular form of protein dietary supply also is a determining factor. So it was assumed that during young stages, larvae need an exogenous supply of free amino acid. Indeed, the stomach is not differentiated in early stages of marine fish larvae and, in absence of hydrochloric acid and pepsin secretion, ingested protein cannot be denatured.
Free amino acids would then be more efficiently absorbed than protein. However, the incorporation of 10 percent essential amino acid mixture in fishmeal-based diets failed to improve growth and survival in sea bass larvae, when compared to larvae fed a diet with the same nitrogenous level brought as whole protein.

Hydrolysate
Protein hydrolysate has long been considered an advantageous protein form for fish larvae. In recent experiments, a 20 percent replacement of fishmeal hydrolysate in a diet resulted in an improvement of the main biological parameters in sea bass larval rearing: growth, survival and skeletal formation.
Hydrolysates are beneficial to larvae, while they generally do not affect juvenile growth, or in some cases, depress it. The beneficial effect of dietary peptides can explain the high cytosolic peptidase activities observed in young larvae. In juveniles, the specific activity of cytosolic peptidases decreases and hydrolysates become less efficient for growth.
Physical aspects of microparticulate diets
Size and manufacture
Diets must be prepared as microparticles of a size appropriate to the size of the larvaes’ mouths. For example, the sizes of the diets used in our experiments at the Fish Nutrition Laboratory in Plouzané, France were 50 to 125 µm for sea bass at first feeding, 125 to 200 µm from day 14 to day 25 and then 200 to 400 µm to day 40.
Microparticles must be well calibrated to minimize waste. Their composition must be homogenous, so ingredients must be incorporated as very fine meal. Some types of meal, such as fishmeal, must be ground and sieved before being included in microparticles.
Nutrient leaching is one of the problems in developing suitable diets for fish larvae. Particles must be water-stable, palatable, and digestible. Diets can be crumbled, or prepared in microbound, microcoated, or microencapsulated form.
Distribution and ingestion
Dietary microparticles must be distributed in large excess. Early-stage larvae have a limited movement, and microparticles must be caught during their descent in the water column. In most species, larvae exhibit a catching behavior and do ingest inert microparticles from the first feeding.
Ingestion rates in sea bream larvae increased from 0.5 to 3 µg per larva per hour at first feeding to 18 to 25 µg per larva per hour for 20-day-old larvae. The authors concluded that larvae ingest microcapsules and living prey at similar rates.
Conclusion
Basic research on animal development and digestive enzymes has led to knowledge that is essential for formulating microparticulate diets and meeting larval-specific nutritional requirements. Now, compound diets for fish larvae exist, and will be improved in coming years.
(Editor’s Note: This article was originally published in the April 2002 print edition of the Global Aquaculture Advocate.)
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Authors
-
Chantal Cahu, Ph.D.
Fish Nutrition Laboratory
Unité Mixte INRA-IFREMER,
BP. 70, 29280 Plouzané, France -
José Zambonino, Ph.D.
Fish Nutrition Laboratory
Unité Mixte INRA-IFREMER,
BP. 70, 29280 Plouzané, France
Tagged With
Related Posts

Aquafeeds
Digestibility of fishery byproducts tested
A study of shrimp feeding demonstrated the digestibility of byproducts prepared from salmon livers, salmon milt, black cod viscera and arrowtooth heads and viscera from Alaskan fisheries processing plants.

Health & Welfare
Diets affect abalone meat quality, shell color
A preliminary study investigated the effects of diet on the meat quality and shell color of Pacific abalone. A test diet and a commercial abalone feed resulted in lower meat protein content compared to that achieved with a diet of Pacific dulse seaweed. The artificial diets also caused the abalone to have yellow or orange shells. The seaweed diet alone resulted in abalone with dark-brown shells. However, a combination of seaweed and either artificial diet improved abalone growth, meat quality and shell color.

Health & Welfare
High supplementation levels of corn gluten meal in turbot diets
In a study, corn gluten meal exerted negative effects on the intestinal health on turbot, including the induction of enteritis in the distal intestine tissue.

Aquafeeds
Is a ‘baby food’ bottleneck looming for aquaculture?
Global aquaculture, particularly farmed shrimp, depends on artemia for hatchery feeds. Supplies meet current needs, but growth will require alternatives.


