Yellow Sea Fisheries Research Institute aims for faster growth rates, higher disease resistance

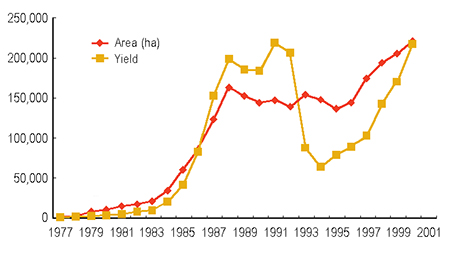
The fleshy shrimp (Fenneropenaeus chinensis) is one of the most important cultured shrimp species in China, where shrimp farming has experienced a tremendous rebound since 1994 (Fig. 1). The species is naturally distributed in a relatively small area between China and Korea.
The shrimp spawn in the coastal waters in spring, grow in the Bohai Sea and Yellow Sea, and in late autumn migrate to deep waters in the East China Sea to over-winter. With its multiple wild populations, the species has a relatively rich genetic diversity and is suitable for genetic improvement using selective breeding and molecular-marker-assisted breeding.
Breeding objectives
At the Yellow Sea Fisheries Research Institute in Qingdao, China, the main objectives for genetic improvement work on F. chinensis are to produce new strains or varieties with faster growth rates, higher disease resistance, and better quality. Institute researchers originally used mass-selection methods, but are currently carrying out selection studies assisted by molecular markers with two populations. The first is a faster-growth population that has been selected since 1997, with selection for five generations completed in 2001. The second is a population selected for disease resistance since 1998, with four generations now selected.
Selection for faster growth
The initial population for faster growth selection was comprised of wild-caught animals from the Yellow Sea, with subsequent generations selected from grow-out ponds. Selection for each generation involved two steps.
The first step was carried out in late autumn. The selection index for growth rate was 1 to 3 percent, and the top males and females were selected before mating. The second selection was done in early March before spawning. In general, about 300 animals were chosen from the first selected and over-wintered population, and used as broodstock for the next generation. Among others, selection criteria included that the animals were free of White Spot Syndrome Virus (WSSV).
Procedures
WSSV-free spawners and postlarvae were used for faster growth selection. Natural seawater was sand-filtered and chlorinated at 5-10ppm. To prevent oral transmission of WSSV, artificial feeds were used, with all live feeds (including artemia, rotifers, and others) tested before use. Indoor, 50-cubic-meter tanks were used for breeding and over-wintering. Grow-out culture was carried out in 0.27-hectare, 2-meter-deep ponds. Photosynthetic bacteria were added to ponds during grow-out to improve water quality.
Selection for disease resistance
The initial population for disease-resistance selection originated with survivors from ponds infected with WSSV, where most shrimp died but some grew normally. Mated females were generally selected directly from WSSV-infected ponds using a selection index of less than 10 percent.
Development of diagnostic kits
Dot blot and polymerase chain reaction diagnostic kits were developed for WSSV diagnostics. The genetic variation among the first three generations of disease-resistant shrimp was analyzed by amplified fragment length polymorphism (AFLP). Four primer pairs – E33M48 (AAG+CAC), E45M54 (ATG+CCT), E35M61 (ACA+CTG), and E39M61 (AGA+CTG) – were used, and 10 individuals were randomly sampled for each generation.
Larger animals
Positive responses were observed in both the growth rate selection and disease resistance selection. Under similar culture conditions, stocking densities, and culture periods, both the average and range of body length for successive generations of selected animals rose over time (Table 1).
Qingyin, Shrimp body length during selective breeding, Table 1
| Selected Shrimp | Sample Date | Average Body Length (cm) | Body Length Range (cm) |
|---|
Selected Shrimp | Sample Date | Average Body Length (cm) | Body Length Range (cm) |
|---|---|---|---|
| Second generation | October 1998 | 12.13 | 9.3-14.2 |
| Third generation | October 1999 | 13.29 | 9.7-15.5 |
| Fourth generation | October 2000 | 13.59 | 11.0-16.4 |
| Fifth generation | October 2001 | 15.10 | 12.9-18.3 |
WSSV challenge
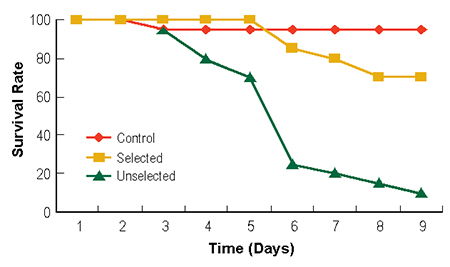
After selection of the disease-resistant population for three generations, survival rates reached 70 percent after a nine-day challenge test with diets prepared using WSSV-infected shrimp. The survival rate of the unselected population after feeding with the same diet was only 10 percent (Fig. 2).
In the viral challenge test, the third generation of shrimp selected for disease resistance showed higher survival rate than unselected shrimp. In the first three days, there were no significant differences between the survival rates. In the next three days, 80 percent of the unselected shrimp died with obvious WSSV symptoms, while only 20 percent of the selected shrimp died. On day 9, only 10 percent of the unselected shrimp survived, while 70 percent of the selected group survived the challenge.
Grow-out results
Culture experiments showed that using the selected WSSV-free postlarvae could significantly decrease shrimp mortality during grow-out and reduce economic losses due to diseases. Of a total 21 experimental ponds stocked with selected WSSV-free PL, only one pond tested positive for WSSV in 1999, while four of 14 control ponds tested positive and shrimp died before normal harvest.
Polymorphism and heterozygosity
Institute studies showed that the rate of polymorphism and heterozygosity of the selected population declined following the advance of selection, as shown by 281 AFLP markers. Thirty of the markers were polymorphisms. Table 2 shows the changes of heterozygosity and polymorphic loci during the selective breeding from first to third generations. Between the first and third generations, a reduction of about one-fourth in the polymorphism loci was detected.
Qingyin, Changes in genetic parameters during selective breeding, Table 2
| Selected Shrimp | Heterozygosity | Polymorphic Loci P95 (%) |
|---|
Selected Shrimp | Heterozygosity | Polymorphic Loci P95 (%) |
|---|---|---|
| First generation | 0.1078 | 36 |
| Second generation | 0.1000 | 33 |
| Third generation | 0.0964 | 27 |
Marker frequencies as genetic tags
The changes of some marker frequencies could be used as genetic tags for each generation. Thirteen markers detected in the first generation could not be checked in the second or third generations, while 10 new markers appeared in the second and third generations.
Some markers were detected in all samples in the second generation, but only one in the first generation and none in the third generation, which could be used as the tags for the second generation (Table 3). Based on these results, the changes of marker frequencies from generation to generation might be used as potential genetic markers for selected traits.
Qingyin, Genetic tags and their frequencies, Table 3
| Selected shrimp | E33M4828 | E33M4878 | E33M4846 | E35M6153 | E39M5541 | E45M5412 |
|---|
Selected shrimp | E33M4828 | E33M4878 | E33M4846 | E35M6153 | E39M5541 | E45M5412 |
|---|---|---|---|---|---|---|
| First generation | 30 | 20 | 10 | 10 | 0 | 10 |
| Second generation | 0 | 0 | 10 | 100 | 20 | 20 |
| Third generation | 0 | 0 | 90 | 0 | 100 | 80 |
Conclusion
Ongoing research on the genetic improvement of F. chinensis has achieved promising results, but it is still at an early stage. Further development and strengthening of the shrimp-farming industry urgently require new shrimp lines characterized by faster growth and stronger disease resistance. Selective breeding, especially molecular marker-assisted selection, will play an important role in this strategy.
(Editor’s Note: This article was originally published in the February 2002 print edition of the Global Aquaculture Advocate.)
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Authors
-
Wang Qingyin, Ph.D.
Yellow Sea Fisheries Research Institute
Chinese Academy of Fishery Sciences
106 Nanjing Road
Qingdao 266071 China -
Kong Jie, Ph.D.
Yellow Sea Fisheries Research Institute
Chinese Academy of Fishery Sciences
106 Nanjing Road
Qingdao 266071 China -
Professor Li Jian
Yellow Sea Fisheries Research Institute
Chinese Academy of Fishery Sciences
106 Nanjing Road
Qingdao 266071 China -
Huang Jie, Ph.D.
Yellow Sea Fisheries Research Institute
Chinese Academy of Fishery Sciences
106 Nanjing Road
Qingdao 266071 China -
Professor Zhao Fazhen
Yellow Sea Fisheries Research Institute
Chinese Academy of Fishery Sciences
106 Nanjing Road
Qingdao 266071 China
Tagged With
Related Posts

Health & Welfare
China rebuilds fleshy shrimp industry
In China, fleshy shrimp have had much higher market value than other shrimp species. China’s aquaculture scientists and fishery agencies have therefore worked closely with shrimp farmers to rebuild the farming industry for F. chinensis.

Health & Welfare
Evaluating heritability of growth, cold tolerance in Chinese white shrimp
Results of this study showed low heritability and a low correlation between growth and cold tolerance traits for Chinese white shrimp juveniles.

Intelligence
Paradigm shifts in shrimp farming
In the evolution of shrimp farming, white shrimp emerged as the primary species. While some farms in the Americas successfully converted to intensive practices, wide adoption has been limited.

Health & Welfare
A look at aquaculture genomics
Advances in genomics assist aquaculture science by deepening the understanding of adaptation, physiology and quantitative genetics.


