Parasite causes serious disease, economic losses in freshwater fish culture

Recently, an increasing number of producers are showing an interest in the culture of hybrid catfish (female channel catfish x male blue catfish) instead of channel catfish, even though the latter has been the dominant cultured species in the United States for several decades.
Compared to channel catfish, hybrid catfish have been reported to have faster growth, better feed conversion, greater tolerance of low oxygen levels and higher fillet yields. The hybrids are also more resistant to the bacterial pathogens Flavobacterium columnare, Edwardsiella ictaluri and Aeromonas hydrophila, but showed no greater resistance to channel catfish virus and proliferative gill disease than channel catfish.
Ich parasite
Ichthyophthirius multifiliis is a ciliated protozoan commonly referred to as ich. The parasite causes serious disease in freshwater fish that leads to heavy economic losses in aquaculture. The parasite has a life cycle consisting of an infective theront, a parasitic trophont and a reproductive tomont.
Chemical treatment of ich infection is difficult after the parasite penetrates the fish skin and gills. Vaccination against the parasite is an alternative to chemical treatments, since fish that survive an ich infection acquire immunity against reinfection by the parasite.
Cohabitation challenge
Since limited information is available on the immune protection of hybrid catfish against ich, the authors performed a study to compare the serum antibody concentrations, parasite infection levels and host protection in immunized hybrid catfish and channel catfish. Disease-free catfish were initially obtained for the study from the Catfish Genetic Research Unit of the United States Department of Agriculture Agricultural Research Service in Stoneville, Miss., USA.
The authors conducted the trial using a cohabitation challenge method in which hybrid and channel catfish occupied the same tanks. Cohabitation is considered one of the best models for evaluation of protective immunity, since two fish species are held in the same rearing unit under the same culture conditions, thereby decreasing the chance for variation among experimental units.
Hybrid catfish and channel catfish can be differentiated easily when they are alive, but not after death. To avoid misidentification, 140 channel catfish were marked with calcein before the trial. Calcein is a green-fluorescent dye that binds to calcium-rich tissues, such as fins and bones. Upon binding, an increase in fluorescence can be observed under ultraviolet light.
When fish showed visible white spots five days after challenge with ich theronts, five hybrid and five channel catfish in each aquarium were sampled to determine infection by the number of white spots on the body surface of each fish.
Immunization study
Twelve tanks with 10 hybrid and 10 channel catfish per tank were assigned for the trial. The mean body weight was 10.7 ± 1.3 grams for hybrid catfish and 10.5 ± 1.0 grams for channel catfish. These fish were divided into four triplicate groups and immunized as follows: immersion at a dose of 10,000 theronts/fish followed by daily formalin treatment for five days, intraperitoneal (I.P.) injection at a dose of 10,000 live theronts/fish, I.P. injection with 5 percent bovine serum albumin (BSA) and a non-immunized control.
Six hybrid catfish and six channel catfish were sampled from each immunized group 14 and 21 days after immunization to collect blood serum and determine the antibody levels against ich by an immobilization assay. In the assay, increasing dilutions of the serum determined the antibody level (expressed as immobilization titer) at which the parasite theronts ceased swimming and aggregated into theront masses.
After 21 days of immunization, water volume was adjusted to 10 L in each of the 12 tanks. Theronts were added to each tank at a dose of 10,000 theronts/fish. Then flowing water was resumed, and mortality of fish in each tank was recorded daily for three weeks after theront exposure for one hour.
Immune responses
Both hybrid and channel catfish showed high antibody levels 21 days following exposure to live theronts by immersion or I.P. injection (Fig. 1). The immobilization titers ranged 7,000 for hybrid catfish to 7,600 for channel catfish when immunized by immersion. Similarly, high immobilization titers were detected for hybrid catfish (7,700) and channel catfish (8,100) when immunized by I.P. injection with live theronts. No anti-ich antibody was detected in non-immunized fish.
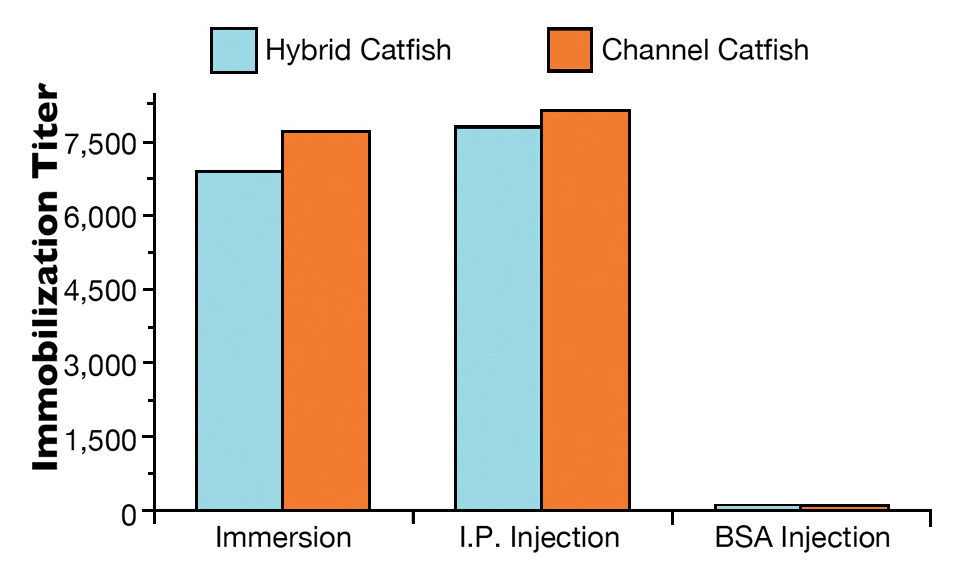
No or light infection below 50 trophonts/fish was observed in fish immunized by immersion or injection. There was no statistical difference in parasite infection level between the immunized hybrid catfish and channel catfish. In fish that received BSA injections and the non-immunized control groups, all hybrid catfish and channel catfish showed heavy infection levels over 100 trophonts/fish.
Immune protection
All hybrid catfish and 97 percent of the channel catfish immunized with live theronts by immersion survived the theront challenge (Fig. 2). For the fish immunized by I.P. injection, 97 percent of the hybrid catfish and 90 percent of the channel catfish survived the theront challenge. Only 30 percent of the hybrid catfish and 27 percent of the channel catfish survived in the group given BSA injections. All of the non-immunized control catfish died.
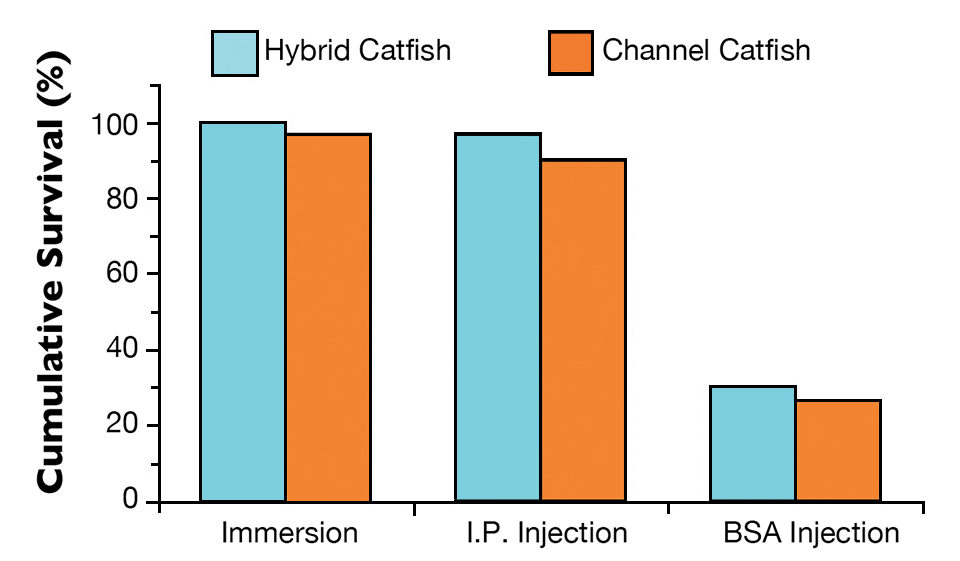
There was no statistical difference in survival between the hybrid and channel catfish. There was a positive correlation between anti-ich antibody levels and fish survival. The fish immunized with live theronts had high anti-ich antibody level and showed high survival.
(Editor’s Note: This article was originally published in the January/February 2014 print edition of the Global Aquaculture Advocate.)
Authors
-
De-Hai Xu, Ph.D.
USDA Agricultural Research Service
Aquatic Animal Health Research Unit
990 Wire Road
Auburn, Alabama 36832 USA -
Phillip Klesius, Ph.D.
USDA Agricultural Research Service
Aquatic Animal Health Research Unit
990 Wire Road
Auburn, Alabama 36832 USA
Tagged With
Related Posts

Aquafeeds
A look at protease enzymes in crustacean nutrition
Food digestion involves digestive enzymes to break down polymeric macromolecules and facilitate nutrient absorption. Enzyme supplementation in aquafeeds is a major alternative to improve feed quality and nutrient digestibility, gut health, compensate digestive enzymes when needed, and may also improve immune responses.

Responsibility
Addressing safety in Latin America’s tilapia supply chain
Over the last decade, the experience gained by many tilapia farmers combined with proficient programs implemented by local governments have significantly improved tilapia production in various Latin American countries like Colombia, Mexico, Ecuador and other important tilapia producers in the region.

Innovation & Investment
Assessing coloration in channel catfish fillets
Because consumers look at color to gauge quality of catfish fillets, the authors developed a digital photography measurement method to assess yellowness.

Health & Welfare
Amino acid supplementation reduces protein levels in pangasius diets
Trials show that supplementation with amino acids could reduce protein levels from a typical 28 percent to 23 percent in pangasius diets.


