Study evaluates the vector function of parasite for Edwardsiella ictaluri
Parasites can cause fish death or poor health by directly damaging organs, utilizing nutrients and thus impacting growth, causing stress and thus reducing immune protection against pathogens, and damaging fish epithelium in skin and gills, aiding bacterial entry into the fish.
Fish mucus covers the epithelium and provides natural protection against pathogens. Studies have demonstrated that parasite injuries act as potential portals of entry for bacterial pathogens. Parasitic infection can enhance bacterial invasion and result in high fish mortality.
There is limited information available on whether parasites act as vectors to transmit pathogenic bacteria to fish. Therefore, a study was conducted to evaluate the vector function of the parasite Ichthyophthirius multifiliis (ich) for the bacterium Edwardsiella ictaluri into channel catfish. Protozoan ich and E. ictaluri are common pathogens of cultured channel fish that can result in high mortality and decreased profits. The life cycle of ich consists of an infective theront, a parasitic trophont and a reproductive tomont.
E. ictaluri attachment
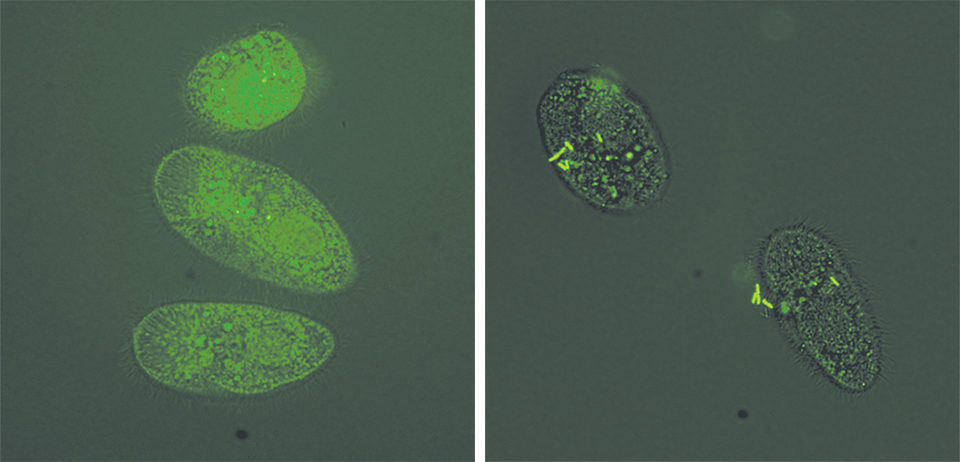
The authors evaluated whether the bacteria could attach to ich theronts using green fluorescent-tagged E. ictaluri. Nine tubes were filled with theront solution, and the tagged E. ictaluri were added at concentrations of 0 colony-forming unit, 4 x 103 CFU and 4 x 107 CFU/mL.
The theronts were exposed to E. ictaluri for one hour in the tubes used for each E. ictaluri concentration. At the end of the exposure, 1 percent formalin was added to each tube to fix the theronts for 30 minutes.
Theronts were harvested after washing three times with sterile water, then suspended in 0.5 ml of sterile water in flow cytometer tubes. The number of theronts carrying fluorescent E. ictaluri was counted for each sample using a flow cytometer. Theronts without E. ictaluri exposure were included as negative controls.
The control theronts showed no bacteria, but many theronts exposed to E. ictaluri were shown to carry fluorescent bacteria. Theronts (24 and 40 percent) showed fluorescent bacteria after exposure to E. ictaluri at 4 x 103 and 4 x 107 CFU/mL, respectively (Table 1).
Hai Xu, Numbers of ice throngs, Table 1
| Concentration of E. ictaluri | Theronts Counted | Fluorescent Theronts* | Theronts Positive for E. ictaluri |
|---|---|---|---|
| 0 CFU/mL | 931 | 53 | 5.7% |
| 4 x 103 CFU/mL | 992 | 233 | 23.5% |
| 4 x 107 CFU/mL | 956 | 321 | 39.0% |
Table 1. Numbers of ich theronts positive for E. ictaluri one hour after exposure.
Tomont exposure
To evaluate whether tomonts exposed to E. ictaluri produced theronts carrying E. ictaluri, the authors added 300 tomonts per well to duplicate six-well plates. E. ictaluri were added to the wells at concentrations of 0, 4 x 105 and 4 x 107 CFU/mL for a two-hour exposure.
The bacterial suspension and unattached tomonts were removed from each well. Then fresh water was added to each well to wash the attached tomonts and remove suspended bacteria. After washing, 30 mL of fresh tank water was added to each well and incubated at 22 ± 2 degrees C. One plate was sampled four or 24 hours after exposure to E. ictaluri. The attached tomonts (four hours) or theronts (24 hours) were harvested and viewed with a fluorescent microscope.
No fluorescent bacteria were observed on the control tomonts. All tomonts demonstrated fluorescent bacteria four hours post-exposure to E. ictaluri (Table 2). After 24 hours, most tomonts divided into several hundred infective theronts. Among those theronts, 31 percent and 66 percent were observed to have bacteria attached to their surfaces following tomont exposure to E. ictaluri at 5 x 105 or 5 x 107 CFU/mL (Table 2). E. ictaluri survived and replicated during the tomont division.
Hai Xu, Number for torments, Table 2
| Concentration of E. ictaluri | Hour 4 P/N | Hour 4 % P | Hour 24 P/N | Hour 24 % P |
|---|---|---|---|---|
| 0 CFU/mL | 0/31 | 0 | 0/261 | 0 |
| 5 x 105 CFU/mL | 45/45 | 100 | 31.2 | |
| 5 x 107 CFU/mL | 40/40 | 100 | 77/247 | 66.4 |
N = Number of tomonts or theronts examined
% P = Percentage of tomonts or theronts positive for E. ictaluri
Table 2. Number of tomonts and theronts positive for E. ictaluri.
Fish infection trial
E. ictaluri were added to ich theront solution in three, 1-L beakers at concentrations of 0, 4 x 105 and 4 x 107 CFU/mL. After exposure to E. ictaluri for one hour, theronts were harvested and washed. Six, 2-L beakers each were filled with 1 L of water and 30 channel catfish fingerlings weighing 0.3 ± 0.1 g. The theronts exposed to various concentrations of E. ictaluri were added to each beaker at 1,000 theronts/fish with two beakers for each treatment. Five fish were sampled from each beaker four hours and one day after theront exposure. Tissue from each sample was grown in bacterial medium for 24 hours to examine the presence of E. ictaluri.
Approximately 60 percent and 90 percent of the fish exposed to theronts treated with 5 x 105 E. ictaluri/mL showed fluorescent bacteria at four hours and one day (Table 3). All fish were positive for E. ictaluri four hours after exposure to theronts treated with 5 x 107 E. ictaluri/mL. Two days post-exposure, cumulative fish mortalities were 36.7 percent, 40.0 percent, and 60.0 percent for fish exposed to theronts only, theronts treated with 5 x 105 E. ictaluri/mL and theronts treated with 5 x 107 E. ictaluri/mL, respectively.
Hai Xu, Number of fish positive, Table 3
| Concentration of E. ictaluri | Hour 4 P/N | Hour 4 % P | Hour 24 P/N | Hour 24 % P |
|---|---|---|---|---|
| 0 CFU/mL | 0/10 | 0 | 0/10 | 0 |
| 5 x 105 CFU/mL | 6/10 | 60 | 9/10 | 90 |
| 5 x 107 CFU/mL | 10/10 | 100 | 10/10 | 100 |
N = Number of fish examined
% P = Percentage of fish positive for E. ictaluri
Table 3. Number of fish positive for E. ictaluri after exposure to theronts treated with E. ictaluri.
Results of this study demonstrated that ich can vector E. ictaluri into channel catfish. Understanding the potential ability of parasites to vector bacterial disease is important to fish farmers and health managers particularly because parasites introduced via wild fish or fish from other farms could concomitantly involve the introduction and/or transmission of microbial disease agents.
(Editor’s Note: This article was originally published in the November/December 2012 print edition of the Global Aquaculture Advocate.)
Authors
-

De-Hai Xu, Ph.D.
USDA Agricultural Research Service
Aquatic Animal Health Research Laboratory
990 Wire Road
Auburn, Alabama 36832 USA[118,111,103,46,97,100,115,117,46,115,114,97,64,117,120,46,105,97,104,101,100]
-

Craig Shoemaker, Ph.D.
USDA Agricultural Research Service
Aquatic Animal Health Research Laboratory
990 Wire Road
Auburn, Alabama 36832 USA -
Phillip Klesius, Ph.D.
USDA Agricultural Research Service
Aquatic Animal Health Research Laboratory
990 Wire Road
Auburn, Alabama 36832 USA
Related Posts

Health & Welfare
AHPN inferences based on behavior of vibrio bacteria
Vibrio parahaemolyticus, a strain of which is the cause of acute hepatopancreatic necrosis (AHPN), has both virulent and benign strains. This strain colonizes the stomachs of shrimp by the formation of a biofilm, which protects it from antibiotics and other potential treatments.

Health & Welfare
A comprehensive look at the Proficiency Test for farmed shrimp
The University of Arizona Aquaculture Pathology Laboratory has carried out the Proficiency Test (PT) since 2005, with 300-plus diagnostic laboratories participating while improving their capabilities in the diagnosis of several shrimp pathogens.

Health & Welfare
A holistic management approach to EMS
Early Mortality Syndrome has devastated farmed shrimp in Asia and Latin America. With better understanding of the pathogen and the development and improvement of novel strategies, shrimp farmers are now able to better manage the disease.

Responsibility
A look at unit processes in RAS systems
The ability to maintain adequate oxygen levels can be a limiting factor in carrying capacities for RAS. The amount of oxygen required is largely dictated by the feed rate and length of time waste solids remain within the systems.


