Experiments conducted at IFREMER facility in Tahiti

Most disease problems in shrimp production are complex and still poorly understood. Sustainable management strategies and regulations restrict the number of drugs available to treat pathogens. Contrary to agriculture, the economics of drugs in aquaculture are not appealing for the pharmaceutical industry to invest in the development of novel, acceptable therapeutics. Vaccines are likely to be ineffective in crustaceans, which lack a specific immune system similar to that of vertebrates. Therefore, shrimp aquaculturists must consider seedstock quality, husbandry procedures, and healthy nutrition as the major tools to control disease.
Improving health via feed
Current nutritional research efforts are directed towards the identification of requirements for key nutrients affecting the health and immunology of shrimp, such as vitamin C and E, phospholipids, essential fatty acids, trace minerals and carotenoids.
One of the most promising areas of development for strengthening the non-specific defense mechanisms and protect shrimp against disease is the administration of immunostimulants. Various compounds have been identified, mostly derived from the cell envelope of microorganisms, such as polysaccharides, lipoproteins, and lipopolysaccharides. Preliminary results indicated that the efficacy of various commercially available immunostimulants to improve stress and/or disease resistance of fish and shrimp strongly depends on the type of the product, and on the supply of adjuvant nutrients that are essential to support the buildup of the immune system. Therefore, the design of specialty premixes to improve disease resistance is based on the selection of the appropriate immunostimulants in combination with a balanced supply of key nutrients to support the enhancement of the immune system.
Experimental conditions, design
Progress in research on disease control of shrimp is hampered by methodological difficulties to assess health status, and limited knowledge of crustacean immunology. In our study, the effect of an immuno-stimulating specialty premix on disease resistance of Penaeus stylirostris was evaluated using haemolymph parameters (ex vivo) as well as a challenge test (in vivo).
Experiments were conducted at the IFREMER Centre Océanologique du Pacifique in Tahiti. Juvenile shrimp (Penaeus stylirostris, 5.1 ± 0.5 grams) were collected from a commercial pond and transferred to the laboratory, where they were maintained in 500-l tanks (70 shrimp per tank) and fed a local commercial feed until the start of the trial.
For each treatment, two groups of animals were stocked:
- Animals used for the analysis of haemolymph parameters: 12 shrimp per 200-liter tank (five tanks = 60 shrimp per treatment).
- Animals used for the challenge test: 50 shrimp per 500-liter tank (three tanks = 150 shrimp per treatment).
The experiment was performed at 26 to 28 degrees-C and 35 ppt salinity. Individual growth was followed during the trial by tagging 45 shrimp per treatment in the 500-liter tanks. Shrimp were fed on the experimental feeds during 21 days prior to the extraction of haemolymph or exposure to the challenge test.

Feed preparation, performance
Experimental feeds were produced by cooking-extrusion. A high performance formulation served as control feed (40 percent crude protein per 8 percent crude fat), in which 1 percent of filler (cellulose) was replaced with an immunostimulating premix (INVE Aquastim-S, Table 1).
Coutteau, Formulation of experimental diets, Table 1
| Raw Material | Control | Aquastim-S |
|---|
Raw Material | Control | Aquastim-S |
|---|---|---|
| Standard fishmeal (72% CP basis) | 33.9 | 33.9 |
| Shrimp head meal | 5.0 | 5.0 |
| Defatted soybean meal | 11.0 | 11.0 |
| Wheat gluten | 2.0 | 2.0 |
| Wheat flour | 16.2 | 16.2 |
| Wheat middlings | 22.6 | 22.6 |
| Fish oil | 1.3 | 1.3 |
| Shrimp premix* | 5.0 | 5.0 |
| Cellulose (filler) | 3.0 | 2.0 |
| INVE Aquastim-S 10-00** | 1.0 | |
| Total | 100.0 | 100.0 |
** Specialty concentrate providing blend of selected immunostimulants and nutrients.
Table 1. Formulation of experimental diets (% of diet).
Shrimp activity was excellent through-out the trial and no mortality was observed during the three-week feeding period. Individual weight gain, measured on tagged shrimp, averaged 3.6 to 3.9 grams over the three-week period (~1.2-1.3 grams per week). Average weight gain was 9 percent higher in the shrimp fed the Aquastim-S supplemented feed, compared to the control group. Similarly, Sung et al. (1994) showed growth enhancement due to immunostimulation in postlarval tiger shrimp (P. monodon).
Haemolymph parameters
The molting stage was determined under the binocular microscope using the method of Drach and Tcherni-govtzeff (1967). Haemolymph was extracted between the last cephalothorax segment and the first abdominal segment using a 1-ml sterile syringe, and only from animals in intermolting stage (stage C or D0) to avoid interference of molting stage.
Total haemocyte count
Total numbers were counted in microtiter plates under an optical microscope without discrimination of the three types of haemocytes using the method of Le Moullac et al. (1997).
Haemocyte phagocytosis activity
Phagocytosis is an important component of the immune system of shrimp, responsible for the elimination of foreign bodies in the shrimp haemolymph. It generates a liberation of free radicals during the so-called “respiratory burst,” which results in a strong microbicidal action. The color change of Nitro Blue Tetrazolium (NBT) is used to evaluate phagocytosis activity through the quantification of the anion superoxyde O2–, which is the first product released during the respiratory burst. The oxidation of NBT (yellow) to formazan (blue) is a measure of the oxidative activity of the haemocytes. The NBT activity of the haemocytes is measured as such (basal NBT activity), or after immunostimulation of the haemocytes in vitro by exposure to an immunostimulant (stimulated NBT activity) following the method of Secombes (1990).
Effect of feed on haemolymph parameters
The total count of haemocytes circulating in the haemolymph showed a high variability between individual shrimp (Table 2). Nevertheless, the total haemocyte count was significantly lower in the Aquastim-S treatment compared to the control group. Immunostimulation may decrease the concentration of circulating haemocytes in the haemolymph, due to infiltration of haemocytes in immunostimulated tissues and organs.
Coutteau, Evolution of total haemocyte count, Table 2
| Feed | Number Shrimp | Average | Standard Error |
|---|
Feed | Number Shrimp | Average | Standard Error | |
|---|---|---|---|---|
| Day 0 (initial) | Commercial feed | 24 | 36,250 | 2352 |
| Day 21 | Control | 32 | 44,506 a | 2170 |
| Day 21 | Aquastim-S | 26 | 32,969 b | 2605 |
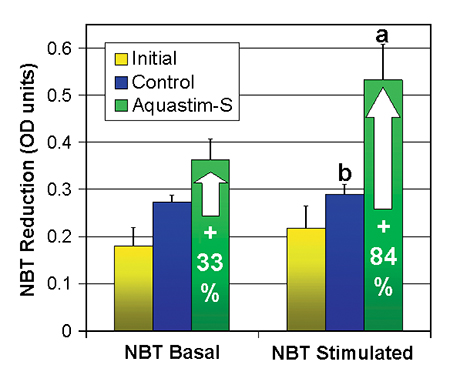
The microbicidal activity of the hae-mocytes, measured as basal as well as stimulated NBT activity, increased for both treatments during the trial compared to the initial value for shrimp fed the commercial feed. The boost of phagocytosis activity was most pronounced in the Aquastim-S group, showing a twofold increase of basal NBT and a 2.4-fold increase of stimulated NBT compared to the initial values (an increase of 33 percent and 84 percent compared to the control feed). The ratio of stimulated NBT per basal NBT, which is a measure of the microbicidal capacity of the haemocytes, increased from 1.1 to 1.5 due to the supplementation of Aquastim-S to the feed.
Challenge test
An experimental infection was performed with a highly virulent bacterial strain of Vibrio penaeicida (AM101). Shrimp were immersed during 2 hours in 10 liters of seawater containing 105 CFU per milliliter of AM101, a dosage pre-tested to target appropriate mortalities in the challenge. Control groups received the same treatment as the challenged shrimp but were not exposed to bacteria during the immersion. After the experimental infection, the shrimp were rinsed with seawater and transferred to tanks containing 25 shrimp per 100 liters of prefiltered seawater (1 µm). The water was continuously aerated and temperature kept at 24 to 26 degrees-C. Mortality was determined three times per day during five consecutive days. Feeding was continued during the follow up period. Infected groups were run with four tanks (~100 shrimp) per treatment, whereas non-infected control groups were run with two tanks (~50 shrimp) per treatment.
Sensitivity
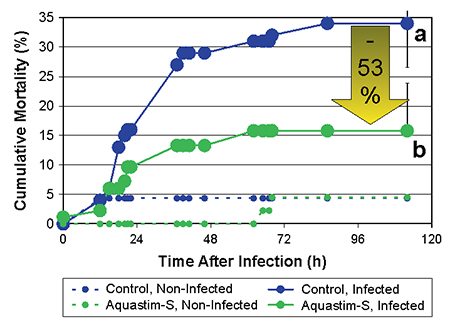
The sensitivity to the challenge test was expressed by the total mortality observed during five days post-infection and the cumulative mortality index, which was calculated as the sum of cumulative mortality observed over the duration of the follow up period. The cumulative mortality index takes into account the total mortality as well as the spread of mortality over time.
Effect of feed on challenge mortality
A limited mortality was observed in the non-infected groups during the follow up period of five days (5 percent in both groups, Fig. 1). After five days, the bacterial infection resulted in a total mortality of 16 percent and 34 percent in the Aquastim-S and control group, respectively (a reduction of mortality with 53 percent due to the feed supplementation, Fig. 2). Similarly, the cumulative mortality index was significantly higher in the control group compared to that of the Aquastim-S group (377±77 and 190±78, respectively).
Conclusion
Our results demonstrated that nutrition is an effective tool to enhance health status of penaeid shrimp. The supplementation of a blend of selected immunostimulants and nutrients to a high quality feed for Penaeus stylirostris resulted in an enhancement of immune competence and resistance to disease after three weeks of feeding, as indicated by:
- A reduction of the total number of haemocytes in circulation in the haemolymph (with 26 percent compared to the control group)
- A boost of the phagocytosis activity of the haemocytes (33 percent and 84 percent increase of basal NBT and stimulated NBT activity, respectively, compared to the control group)
- Improved survival in a challenge test (53 percent less mortality compared to the control group following an experimental infection with Vibrio penaeicida).
Note: Cited references are available from the first author.
(Editor’s Note: This article was originally published in the October 2001 print edition of the Global Aquaculture Advocate.)
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Authors
-
Peter Coutteau
INVE Technologies nv
Aquaculture Division – Farm Nutrition
Oeverstraat 7
B-9200 Baasrode, Belgium -
S. Ceulemans
INVE Technologies nv
Aquaculture Division – Farm Nutrition
Oeverstraat 7
B-9200 Baasrode, Belgium -
L. Chim
IFREMER
Centre Océanologique du Pacifique Tahiti
French Polynesia -
D. Saulnier
IFREMER
Centre Océanologique du Pacifique Tahiti
French Polynesia -
P. Lemaire
IFREMER
Centre Océanologique du Pacifique Tahiti
French Polynesia
Related Posts

Health & Welfare
Blue shrimp quarantined in New Caledonia genetic variability program
To improve the sustainability of their breeding programs, shrimp producers in New Caledonia established a program with certified SPF blue shrimp.

Responsibility
Floating skimmers improve water quality in tanks, ponds
In aquaculture, foam fractionation involves the use of a protein skimmer to remove dissolved organic compounds from aquaculture systems.

Health & Welfare
French program studies selective breeding in oyster spat
Oyster spat survival will be a selected trait for a full-scale, family-based selective breeding program being discussed with the French oyster industry.

Health & Welfare
Preventing melanosis in shrimp
The black spots of melanosis quickly arise in harvested shrimp from enzymatic reactions in shrimp tissue or external stressors.


