Both biocides tested had inhibitory effect on spore extrusion rate
Microsporidia is a vast phylum [taxonomic rank for organisms sharing a similar body plan] composed of more than 1400 species of eukaryotic [organisms whose cells have a nucleus enclosed within a nuclear envelope], unicellular and intracellular fungi related parasites. They are able to infect a wide variety of aquatic hosts and become a pathogen of high relevance in the aquatic environment where it has been an emergent problem. In 2004, an unnamed microsporidian was first reported in the hepatopancreas of black tiger shrimp (Penaeus monodon) specimens affected by slow growth in Thailand.
However, it was not until 2009 when this pathogen was fully characterized and identified as Enterocytozoon hepatopenaei (EHP), and the very serious disease it caused in shrimp as hepatopancreatic microsporidiosis (HPM). This pathogen has been found in black tiger shrimp, whiteleg shrimp (P. vannamei) and possibly kuruma shrimp (Marsupenaeus japonicus), and has been reported in some countries with major shrimp aquaculture industries, such as China, Vietnam, Indonesia, Brunei, India, Malaysia, the Australasian region (suspected), Venezuela and Mexico (possibly different species).
EHP can be transmitted horizontally via cohabitation with infected specimens, cannibalism, contaminated water, soil or equipment. The microsporidia life cycle is divided into three main stages, including proliferative (merogeny), spore production (sporogeny) and the infective stage (mature spore). The spores are generally of ovoid shape, small in size of ~1 to 4 μm and containing a polar filament of four to five coils that can be extruded and used to perforate host cells and inject infective materials. They can be found in the hepatopancreatic epithelial tubules. For many years, it has been reported that the main sign of EHP infection is retarded growth in affected shrimp and without any visible external signs; however, these infections can be a risk factor for opportunistic pathogens to be able to initiate secondary infections.
https://www.aquaculturealliance.org/advocate/white-feces-syndrome-shrimp-predictor-ehp/
As the shrimp farming industry intensifies and increases its production, diseases continue playing a major role in its success. A widely used approach to treat diseases in aquatic farms is the application of chemicals under prevention or remedy strategies to control the disease impacts and in-pond environment. In the case of EHP, there was confusion on the causative agents of EHP, which was usually linked with other diseases like acute hepatopancreatic necrosis disease (AHPND), also known as Early Mortality Syndrome (EMS).
From the previous results of a co-infection challenge model, several important conclusions were made on the escalated destruction of hepatopancreatic tissues and susceptibility of shrimp to Vibriospp. causing AHPND/EMS and septic hepatopancreatic necrosis (SHPN).
Additionally, another study found a correlation between EHP and white feces syndrome (WFS) while massive amounts of EHP spores were being quantified in feces of WFS-infected shrimp. Diagnostics of EHP can be via microscopy (wet mount of hepatopancreatic tissue), molecular methods (PCR) and tissue analysis (histology or in situ hybridization).
Development of surface surfactants and drug treatment for EHP is one of suitable methods to control EHP. There is still an opportunity for a vast number of products that can be studied for their suitability to treat and control of EHP infections, such as biocides [chemical substances used to destroy or control harmful organisms]. In this article, the results and observations obtained by studying the biocide effect of two commercial products, Aqua-Protect and Antizol (manufactured by Virbac Vietnam), are presented. The goal of this study was to determine the potential of these two biocidal agents against EHP spore extrusion under laboratory conditions via in vitro studies.
Study setup
Two different batches of EHP-infected shrimp were obtained from two geographically different sources: the Provinces of Pathum Thani (Batch 1) and Chanthaburi (Batch 2) in Thailand. These shrimp were acclimated at temperatures ranging from 28 to 29 degrees-C with constant aeration in the laboratory for 2 days (Service Unit at Centex Shrimp, Mahidol University, Bangkok) before their hepatopancreas were collected and used for EHP spore purification according to the published method of Aldama-Cano et al. (2018). The rate of spore extrusion was measured and reported as 77.0±2.65 percent and 70.3±2.08 percent, respectively, for batches 1 and 2 of the EHP spores.
Preparation of the test solutions of the two commercial biocides was as follows: Aqua-Protect and Antizol were diluted in distilled water to initial 1,000 ppm stock solutions, which were then further diluted to 1, 5 and 10 ppm for Aqua-Protect, and 10, 20 and 40 ppm for Antizol to test on the Batch 1 of the EHP spores. For Batch 2 of the EHP spores, Aqua-Protect was diluted to 1, 5, 10 and 20 ppm, and Antizol was diluted to 10, 20, 40 and 80 ppm working concentrations from the 1000 ppm stock (Table 1). For both batches, the dilutions were prepared 10 to 20 minutes before adding the purified active spores, and the experiment was carried out at room temperature (~25 degrees-C).
Phan, EHP spore extrusion, Table 1
| Biocide compound | Batch 1 of EHP | Batch 2 of EHP |
|---|
Biocide compound | Batch 1 of EHP | Batch 2 of EHP | |||||
|---|---|---|---|---|---|---|---|
| Aqua-Protect | 1 ppm | 5 ppm | 10 ppm | 1 ppm | 5 ppm | 10 ppm | 20 ppm |
| Antizol | 10 ppm | 20 ppm | 40 ppm | 10 ppm | 20 ppm | 40 ppm | 80 ppm |
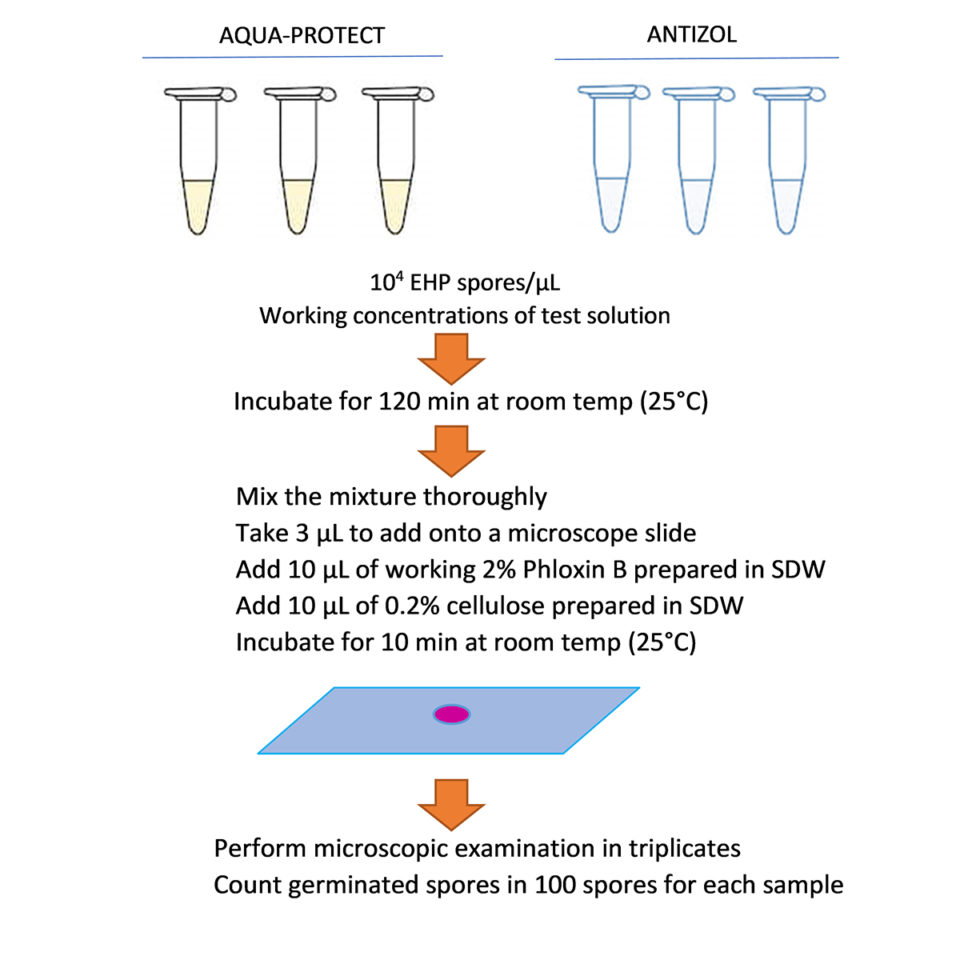
Regarding the spore extrusion assays, the EHP spore inhibition tests were carried out in 100 µL reaction tubes. Each tube was prepared by adding purified EHP spores to each concentration of the diluted products to achieve the final spore concentration of 104 spores/µL. The control tests were performed in sterile distilled water (SDW) instead of test solutions. All reactions were incubated for 120 minutes at room temperature (~25 degrees-C). To calculate the extrusion rate, 3 µL of each reaction was deposited on a glass slide along with 10 µL of 2 percent Phloxin B and 10 µL of 0.2 percent cellulose, after which they were incubated for 10 minutes. One hundred spores were counted and scored as extruded or non-extruded spores. Each reaction was counted under a microscope three times to calculate a mean extrusion rate which was, in turn, divided by the extrusion rate of the corresponding control treatment of each batch to compute the normalized extrusion rate (Fig. 1).
Data were analyzed using one-way analysis of variance (ANOVA) – using SPSS version 22 (IBM). Duncan’s multiple range post-hoc test was applied for multiple comparisons of significant differences between treatments (p < 0.05).
Results and discussion
The in vitro spore extrusion tests were carried out with two batches of EHP spores collected from two different sources of EHP-infected shrimp. The extrusion rates of the control treatments were ~77.0 percent and ~70.3 percent from the first and second batches of spores, respectively (Table 1). These values were used to normalize the extrusion rate of the treated spores in their respective experimental batches.
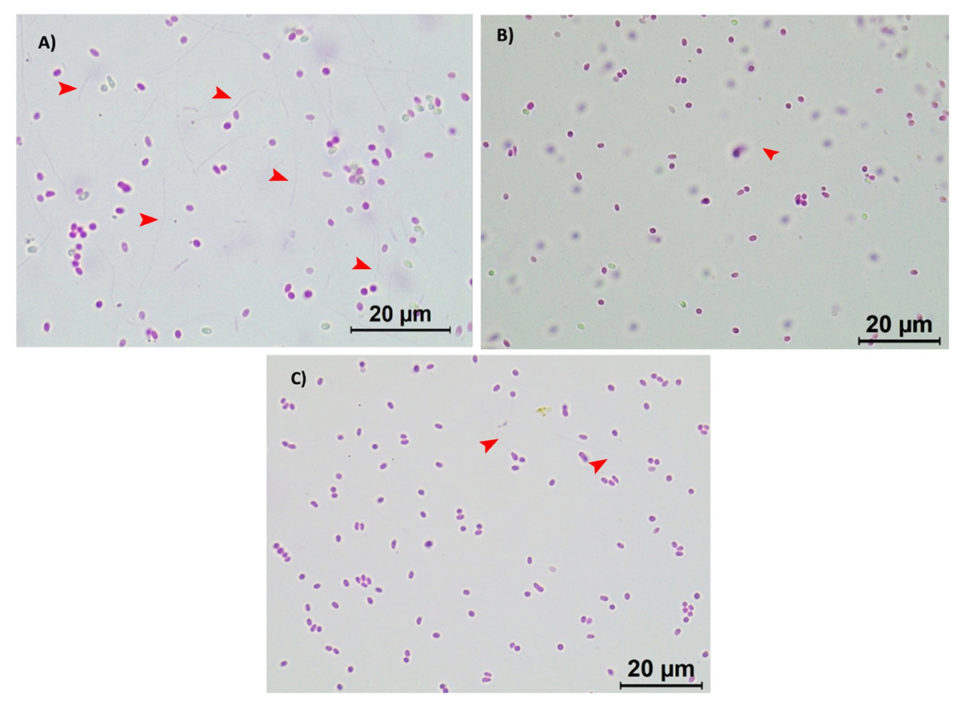
It was observed that both batches of active EHP spores responded to the treatments with the biocides in a similar manner. Under a light microscope (Leica, 100× magnification), a high number of extruded spores can be observed in the control treatment (Fig. 2A; where polar tubes have been extruded demonstrating their active and infective stage), whereas a significant reduction on the polar tube extrusion was observed in the active EHP spores treated with 20 ppm of Aqua-Protect (Fig. 2B) and 80 ppm of Antizol (Fig. 2C). Statistical analysis indicated a significant difference (p < 0.05) in the EHP spore extrusion rates after being treated with the biocides (Table 2 and Fig. 3).
Phan, EHP spore extrusion, Table 2
| Aqua-Protect | Batch 1 | Batch 2 | Antizol | Batch 1 | Batch 2 |
|---|
Aqua-Protect | Batch 1 | Batch 2 | Antizol | Batch 1 | Batch 2 |
|---|---|---|---|---|---|
| Control | 100±2.65a | 100±2.08A | Control | 100±2.65x | 100±2.08X |
| 1 ppm | 59.74±3.61b | 80.57±3.79B | 10 ppm | 9.96±1.15y | 17.06±1.00Y |
| 5 ppm | 38.96±8.66c | 33.18±6.66C | 20 ppm | 14.29±1.00y | 9.00±2.52Y |
| 10 ppm | 36.80±3.51c | 18.96±3.51D | 40 ppm | .36±1.53y | 9.00±2.31Y |
| 20 ppm | N/D | 4.74±0.58E | 80 ppm | N/D | 3.32±1.53Y |
N/D – Not Done. Different superscripts are significantly different from one another in the same column (p < 0.05). Uppercase letters and lowercase letters represent data from each batch of EHP spores tested.
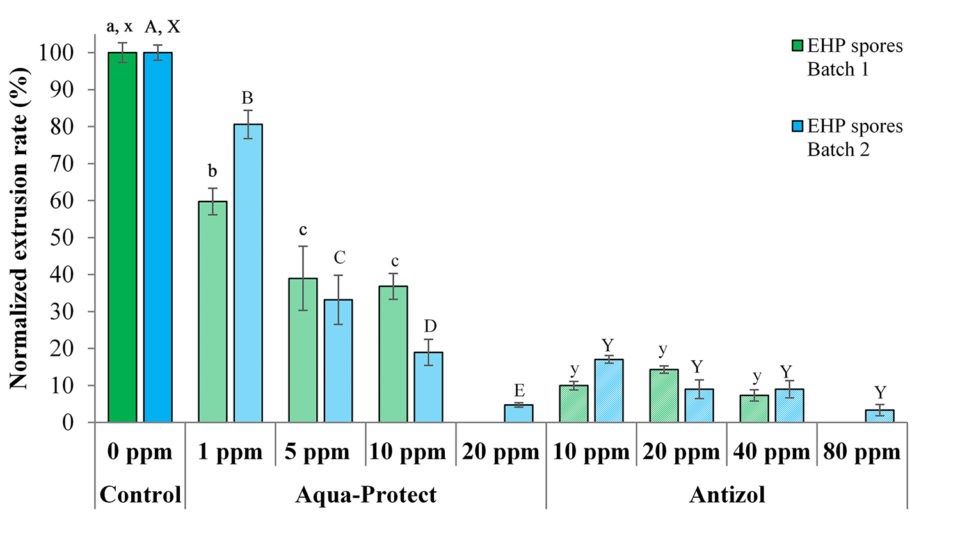
Data from the tests show that the biocides were effective against both batches of EHP spores by inhibiting spore polar tube extrusion after 120 minutes of incubation (Table 2 and Fig. 3). For Antizol, the maximum inhibition rates ranged from 92.6 percent (Batch 1, 40 ppm) to 96.7 percent (Batch 2, 80 ppm), while for Aqua-protect the inhibition rates were from 63.2 percent (Batch 1, 10 ppm) to 95.2 percent (Batch 2, 20 ppm).
Comparing the two products, Antizol was more effective than Aqua-Protect at the same dose of 10 ppm, whereas Aqua-Protect was more effective than Antizol at the dose of 20 ppm (Table 2 and Fig. 3). The inhibitory effect of Aqua-Protect was found to be dose-dependent since an increase in the concentration from 1 ppm to 20 ppm led to a decrease in the extrusion rate. However, the extrusion rate in response to Antizol application did not decrease significantly since the dose increased from 10 ppm.
Conclusion
The results of our study presented here showed that the biocides Aqua-Protect and Antizol had an inhibitory effect on the extrusion rate of EHP spores when treated in vitro under laboratory conditions. While both biocides appear to inhibit extrusion under a strict laboratory setting, it is necessary to consider several factors when optimizing procedures for their use in the field, such as toxicity levels to animals (data are available), application methods/strategies, optimal working concentrations, area or volume of water to be treated and other considerations.
It would also be necessary to determine whether a prophylactic or preventive approach is more suitable than a remedial approach, as most of the chemicals evaluated to possibly fight EHP infections have been recommended to be used in-between crops as a preventive biosafety measure. Once the best approach is determined, the use of disinfectants such as biocides to control EHP infections could become a common practice for both affected and non-affected shrimp farmers.
References available from the corresponding author.
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Authors
-
Khanittha Sang-Ngam
Virbac, Aquaculture Division, France
-
Diva J. Aldama-Cano, Ph.D.
Center of Excellence for Shrimp Molecular Biology and Biotechnology (CENTEX Shrimp)
Mahidol University, Thailand -
Ornchuma Itsathitphaisarn, Ph.D.
Center of Excellence for Shrimp Molecular Biology and Biotechnology (CENTEX Shrimp)
Mahidol University, Thailand -
Philippe Mahl
Virbac, Aquaculture Division, France
-
Phuong Do
Virbac, Aquaculture Division, France
-
Tagged With
Related Posts

Health & Welfare
Challenging Pacific white postlarvae with AHPND
Study results indicate that P. vannamei challenged with AHPND in biofloc had higher survival rates than shrimp challenged in clear water.

Health & Welfare
EHP a risk factor for other shrimp diseases
Laboratory challenges and a case-control study were used to determine the effects of EHP infection on two Vibrio diseases: acute hepatopancreatic necrosis disease (AHPND) and septic hepatopancreatic necrosis (SHPN).

Health & Welfare
Microsporidian impacts shrimp production
Enterocytozoon hepatopenaei (EHP), a microsporidian parasite widely found in Asia and other areas, is impacting aquaculture by severely retarding the growth of cultured shrimp. EHP infects the tubules of the hepatopancreas in shrimp, which damages the organ’s ability to gain nutrition from feed.

Health & Welfare
White Feces Syndrome in shrimp: Predictor of EHP?
Study demonstrates strong association between White Feces Syndrome and Enterocytozoon hepatopenaei in EHP-endemic regions. Biosecurity strategies can minimize the risk of pathogen’s spread in the Americas.


