Ovary development in female triploid shrimp was delayed

Compared with other shellfish and fish, research on chromosome manipulation in shrimp has progressed very slowly due to the shrimp’s particular reproductive characteristics. However, during the last decade, the authors carried out a series of studies on polyploid induction in fleshy shrimp in China. Fleshy shrimp (Fenneropenaeus chinensis) are one of the most important commercial shrimp species in the country.
Polyploid organisms or cells have more than twice the haploid number of chromosomes. Haploid refers to a single set of chromosomes – half the full set of genetic material – present in the egg and sperm cells of animals.
Triploids were produced successfully through physical or chemical treatments, and many batches of triploids have grown to maturity. The reproductive characteristics of triploid shrimp were also studied in detail.
Triploid testing
In the studies, gravid shrimp were obtained from wild populations in the Yellow Sea. These shrimp were maintained in large fiberglass tanks where the photoperiod was controlled to induce animals to spawn during the day. When the animals began to spawn, they were removed and placed into 20-liter containers with seawater in order to gather the fertilized eggs.
The eggs were treated to inhibit polar body I or polar body II with heat shock or 6-dimethylaminopurine (6-DMAP), a reversible inhibitor of the transition to metaphase during the first meiotic cell division. Flow cytometry was used to detect ploidy at the nauplius stage. Hatched larvae were reared following standard methods, and postlarvae were then grown to adults.
During the grow-out, 20 shrimp were collected each month from mid-August to December to determine their ploidy by flow cytometry and select triploid shrimp. The gonads of the experimental animals were dissected out and fixed in Bouin solution for histological study. To observe the morphology of the sperm in triploid shrimp, sampled vas deferens was fixed in 2.5 percent glutaraldehyde buffered with filtered seawater for scanning electronic microscope study.
Normal growth, reproduction inhibited
Triploid shrimp were successfully induced by heat shock or 6-DMAP. The highest triploid level at the nauplius stage reached more than 90 percent. Triploid larvae were able to smoothly pass through various developmental stages and grow normally.
The ovaries of triploid shrimp were much smaller than those of diploid animals at the same stage. The lobes of triploid ovaries were thinner and narrower than those from normal, diploid animals.
Histological studies showed that the germ cells in the ovaries of triploids remained mainly at the oogonia stage, even until the end of December. Very few oocytes were observed in individual ovaries sampled during November and December (Fig. 1). As the triploid shrimp grew out, some germ cells in the ovaries showed a degraded appearance.
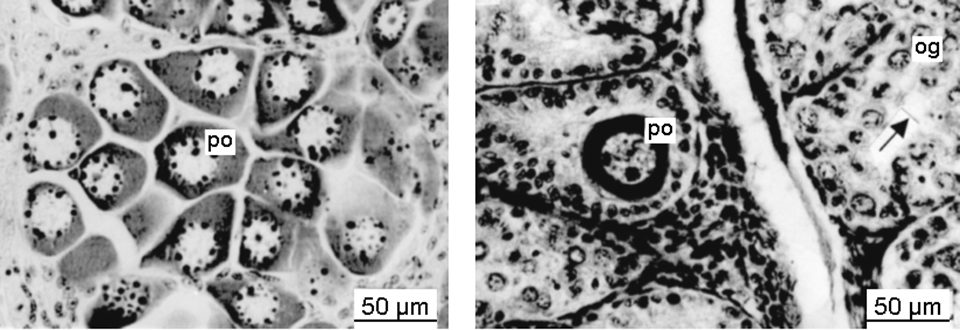
Spermatogonia, primary spermatocytes, and secondary spermatocytes were observed in the testes of triploid shrimp. In some testis lobes, sperm cells were observed sporadically, but the amount of sperm was much less than that in diploid shrimp at the same stage. There were some sperm in the vas deferens and spermatophores of some of the triploid shrimp, but their size was different from normal animals’ sperm.
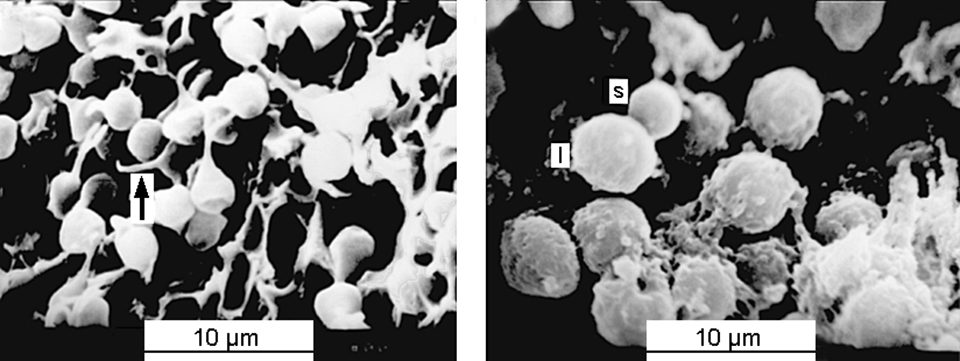
Under the scanning electron microscope, the sperm in vas deferens of triploid shrimp showed different morphology than normal sperm from diploid shrimp. The sizes of normal sperm in the diploids were homologous, and the tapering spikes of the sperm were very apparent. However, in triploid shrimp, the sperm size varied and no spike appeared on these sperm (Fig. 2). The data indicated that triploid shrimp could produce sperm, but all the sperm were abnormal.
There were more female shrimp than males in the triploid population, with the female:male ratio close to 4:1. The study results provided evidence that triploid shrimp are sterile and do not represent a threat to wild shrimp populations.
Conclusion
Triploid fleshy shrimp can be produced by heat shock or 6-DMAP to a level over 90 percent and cultured to adults. In tests, ovary development in female triploid shrimp was delayed. A small amount of sperm was found in male triploid shrimp, but its morphology was abnormal.
(Editor’s Note: This article was originally published in the February 2004 print edition of the Global Aquaculture Advocate.)
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Authors
-
Prof. Jianhai Xiang
Institute of Oceanology
Chinese Academy of Sciences
7 Nanhai Road
Qingdao 266071
P.R. China[110,99,46,99,97,46,111,105,100,113,46,115,109,64,103,110,97,105,120,104,106]
-
Prof. Fuhua Li
Institute of Oceanology
Chinese Academy of Sciences
7 Nanhai Road
Qingdao 266071
P.R. China -
Prof. Linghua Zhou
Institute of Oceanology
Chinese Academy of Sciences
7 Nanhai Road
Qingdao 266071
P.R. China
Tagged With
Related Posts

Health & Welfare
China rebuilds fleshy shrimp industry
In China, fleshy shrimp have had much higher market value than other shrimp species. China’s aquaculture scientists and fishery agencies have therefore worked closely with shrimp farmers to rebuild the farming industry for F. chinensis.

Health & Welfare
Evaluating heritability of growth, cold tolerance in Chinese white shrimp
Results of this study showed low heritability and a low correlation between growth and cold tolerance traits for Chinese white shrimp juveniles.

Intelligence
Paradigm shifts in shrimp farming
In the evolution of shrimp farming, white shrimp emerged as the primary species. While some farms in the Americas successfully converted to intensive practices, wide adoption has been limited.

Health & Welfare
Challenges to commercializing shrimp triploidy
Once satisfactory performance is demonstrated in commercial larval rearing and grow-out, automated induction will finalize the triploidy commercialization.


