Product is an important live feed for shrimp broodstock maturation

Artemia cysts have been used commercially in modern fish and crustacean aquaculture since the 1930s. It is estimated that more than 3,000 metric tons (MT) of artemia cysts are consumed annually by the aquaculture industry. The cysts measure between 250 to 300 microns and, the average weight of an artemia cyst is approximately 0.0066 mg. In Ecuador, shrimp hatcheries that use imported artemia cysts cover the demand for seed of about 300,000 hectares of shrimp farms.
Until around a couple of decades ago, the highest production of quality artemia cysts came from the Great Salt Lake of Utah in the USA. However, due to the great demand for this important live food for hatcheries, new hyperhaline environments are being exploited to obtain artemia cysts in Siberia (Russia), Kazakhstan and China.
But climate change and global warming are altering the hyperhaline conditions of these large lakes around the globe, which is of concern to aquafarmers. This results in more appropriate management techniques used in these lakes, and in reduced consumption of these cysts per million shrimp larvae in shrimp hatcheries around the world. There is also a synthetic product in the market that that could replace artemia cysts in the future, although in my opinion a live feed is better utilized by the larvae in their natural state and from culture.
Artemia, when it develops populations in balanced environments – where it finds the proper balance for its nutrition and stable environmental parameters that avoid population stress – maintains its viviparous reproduction. The zygote develops inside the female artemia, with the live nauplius coming out without the need form a cyst. In this way, all that energy involved in ovoviviparous reproduction is channeled to the production of live nauplii in the environment. From earlier experiments and observations in vitro, we estimated a daily production per female of 18 to 25 nauplii in 24 hours.
Original artemia production project in Peru
In 1994, we developed the Artemia del Pacífico project in the hyperhaline, Virrilá (Sechura) estuary in northern Peru. The initial plan was the production of artemia cysts in artificial ponds. However, we soon discovered aspects of the natural behavior of the artemia population in the area, which led to the production of artemia biomass and its viviparous reproduction. We observed that the population remained in viviparous reproduction, even in areas where salinity exceeded 180 psu. We also observed thousands of migratory and endemic birds at the end of the estuary, and their accumulated excrements, or guano, is consumed by artemia.

From this initial experience in Peru, we obtained very important information that allowed us to learn and increase our knowledge on the ecology of artemia and its viviparous production. For example, with underwater observations of the swarms of artemia (75 percent adults), we demonstrated the aggregation behavior of the population on the pond bottoms. Those individuals closest to the ground maintain the natural form of “inverted swimming,” thus creating an upward current that carries nutrients (guano, bacteria and detritus) to the rest of the population swimming above, which feeds on these. We described this as a gregarious feeding behavior.
Another interesting discovery we made was finding remains of a macroalga (Ulva lactuca) in the digestive tract of very healthy artemia that had been isolated in a concrete tank with saltwater, and where this seaweed had grown on the tank walls. Although artemia do not have chelas or antennas that allow them to cut or grab food (the technical literature considers them as non-selective filtering animals), our observations showed that male specimens can “pinch” with special antennas whose only recognized function – until then – was for copulation, but discovered that they also serve as scissors to prune macroalgae as an additional food source for the rest of the population during the gregarious feeding behavior previously mentioned.
The initial objective of the project changed as it became more productive, and we concentrated on the production of biomass of viviparous artemia instead of cysts. At that time (1994), David Kawahigashi – a scientist working in Hawaii for the San Francisco Bay company – developed the concept of bioencapsulation with enrichment in adult Artemia, to feed shrimp broodstock animals. We combined our experiences and knowledge, and developed the company Artemia del Pacifico in Sechura, Peru, to commercially produce artemia biomass and enriched, frozen artemia to feed shrimp broodstock in hatcheries.
Production of artemia biomass in Ecuador
Since the year 2000, our company, Bioartemia Cía. Ltda., has been developing a program for the commercial production of artemia biomass through viviparous reproduction in the peninsula of Santa Elena, Ecuador. Using the brine conditions of a salt-producing company (Ecuasal), the major producer of salt for human consumption in the country, we follow and adapt to the natural production season of artemia, which vary throughout the year.

The artemia population is maintained in the evaporator ponds, where the salinity is above 100 ups and there are no predatory fish like mullets, guppies or others. The average production of artemia is 200 to 300 pounds per hectare per month. For the harvest, 2-millimeter mesh nets are used, which only capture adult animals. The periodic and directed harvests in places where artemia biomass concentrates allows its populations to have more constant, larger volumes.

In a normal cycle of population growth in natural areas, the artemia biomass can reach overpopulation levels; when this occurs, most of the population dies off and the last survivors change to ovoviviparous reproduction, allowing the population to not go extinct until a new life cycle of natural production begins.
This process causes the population to, overall, decrease over time. In a controlled system like the one we maintain, by making selective extractions/harvests of adults only, new generations of artemia are allowed to have the resources – space and food – so that, in a short term, the population can continue to increase, which allows to provide a rational and sustainable management to our artemia systems.
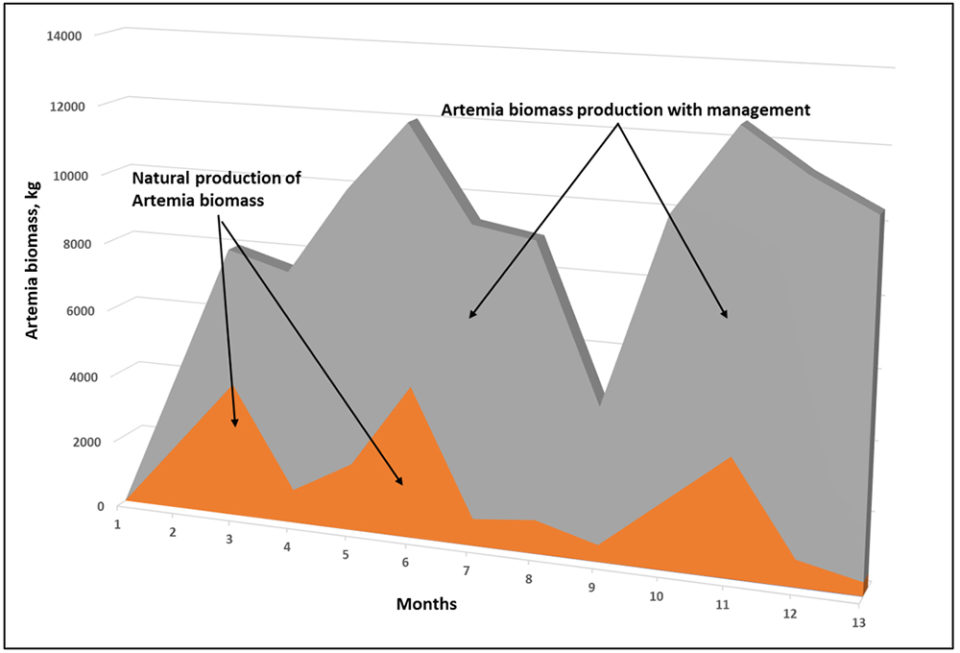
Additionally, we maintain a good production of artemia biomass in 0.25-hectare earthen, recirculating ponds where the water salinity is controlled and maintained between 95 to 110 psu. These improve the conditions in these intensive production ponds with fertilizers, beneficial bacteria and micronized foods, achieving an average of 2,000 to 3,500 pounds per hectare of artemia biomass per month.

The harvested artemia biomass is placed immediately in cleaning systems with filtered water and with salinity between 90 to 110 ups to remove any accompanying fauna such as pupae of aquatic Corixidae insects or Ephydridaepupae. Strong water jets are used to clean the artemia of any adherence from the earthen ponds, and strong aeration complemented with oxygen are required for the cleaning process.
Once the process of cleaning the artemia biomass has been completed, the enrichment process continues, where the animals are immersed for a time in an emulsion containing omega-3 and n-6 fatty acids, the pigment astanxanthin, various vitamins, cholesterol and other nutrients with high levels of absorption and natural attractant.
After the appropriate time of enrichment, a quality control is carried out with observations of the artemia digestive tracts, looking for droplets of the emulsion and the enriching products, in what is known as bioencapsulation. Subsequently, the product is immersed in freshwater disinfected with organic products, excess water is removed and the cleaned and enriched artemia biomass is packaged in 2-pound net and frozen-weight packages.

Biosecurity procedures implemented, continuous screening testing and the artemia processing at high salinities prevents the presence of pathogenic agents like viruses, bacteria and fungi. This product is used mainly to feed shrimp broodstock animals during their sexual maturation in commercial shrimp hatcheries.

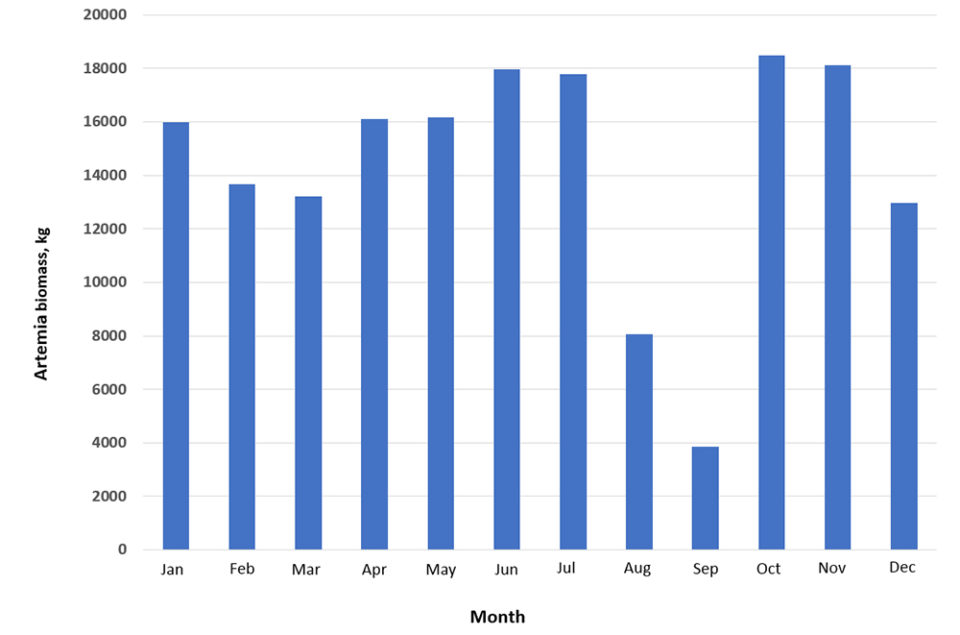
Fig. 2 shows a summary of the last 10 years of our production of artemia biomass in Salinas-Santa Elena, Ecuador. The decline observed annually between August and September coincides with the annual arrival of Phalaropus sp. migratory birds, at reported densities of 40,000 to 60,000 individuals that come to feed during their migratory passage, also taking advantage of the biomass.
Climate is one of the most important factors in the production of artemia biomass. Our area, the Santa Elena Peninsula, has the influence of two marked seasons, the rainy season and the predominant dry season. During periodic El Niño (a sporadic, major weather phenomenon characterized by unusually warm ocean temperatures in the Equatorial Pacific) events, rainfall markedly increases and significantly changing the conditions in the artemia production systems. Moderate rainfall improves primary production by fixing nitrogen from the environment. Extended cloudiness over long periods is detrimental, such as the current period, because it compromises the primary production (amount of light for photosynthesis) and consequently the artemia biomass production decreases considerably.

Perspectives
Artemia is an important component of the broodstock maturation diet for the substantial number of nauplii required to sustain the significant and growing shrimp farming industry in Ecuador.
Climate change and global warming will determine and control the natural production of artemia in the world’s large hypersaline lakes in the coming years. Substitutes for artemia cysts and their nutritional level remain important research priorities. The replacement of artemia cysts, as well as synthetic products, should be considered for alternative live foods such as rotifers and copepods, even with the availability of artemia-enriched metanauplii, also a product of viviparous reproduction.
References available from author.
Author
-
Biol. Jaime Yockteng Fuenzalida
Bioartemia Cía. Ltda.
Santa Elena, Ecuador
Tagged With
Related Posts

Innovation & Investment
Artemia, the ‘magic powder’ fueling a multi-billion-dollar industry
Artemia, microscopic brine shrimp used as feed in hatcheries, are the unsung heroes of aquaculture. Experts say artemia is still inspiring innovation more than 50 years after initial commercialization. These creatures are much more than Sea-Monkeys.

Aquafeeds
Biosecurity protocols needed for shrimp feeds, feeding practices
Shrimp aquafeeds – live, fresh or formulated – should not be an entry point of potential pathogens to the shrimp and/or to their culture systems.

Aquafeeds
Biofloc consumption by Pacific white shrimp postlarvae
The stable isotopes technique with δ13C and δ15N can be used to determine the relevance of different food sources to shrimp feeding during the pre-nursery phase of Litopenaeus vannamei culture. During this trial, different types of commercial feed, microalgae, Artemia sp. nauplii and bioflocs were used as food sources.

Innovation & Investment
Algae innovators aim to freeze out early-stage shrimp losses
A greenhouse in Belgium believes its innovative shrimp feed product, made from freeze-dried microalgae, packs the necessary nutrients for the crustacean’s most vulnerable life stage: the first three days of its life.


