Wastewater treatment and production of valuable fatty acids and tocopherols
Editor’s note: There are nine co-authors in this study but we only list the corresponding author. Please consult the original publication for names and affiliations of all co-authors.
The global growth of fish farming has resulted in serious concerns regarding environmental pollution related to the aquaculture wastewater and over-exploitation of wild fish populations used as fish feed ingredients. Environmentally friendly practices in fish farming and the use of local and low trophic level biomass, e.g., microalgae for feed, may decrease the environmental impacts of fish farming.
Use of recirculating aquaculture systems (RAS) decreases the pollution effect of fish farming and the need for fresh water. Therefore, RAS technology is recognized as a future solution to develop aquaculture practices in several European countries. In RAS, wastewater is treated and a portion of the effluent water is recirculated through the aquaculture system.
In comparison to flow-through culturing systems, the water exchange rate in very intensive RAS is reduced from >50 to <0.1 cubic meters per kg fish feed. Additionally, minimizing feed use (i.e., feed conversion ratio) reduces waste generation in RAS. However, treatment of concentrated sludge containing suspended solids and nutrients, especially phosphorus, increases operating costs.
Decreased use of fish oils in feed diminishes the content of long-chain polyunsaturated fatty acids (LC-PUFAs) in the feed, especially omega-3 fatty acids. Microalgae are a natural source of LC-PUFAs in aquatic food webs and therefore, to avoid deterioration of the nutritional value of farmed fish, interest in replacing LC-PUFA rich fish oils with microalgae is rising.
This article – summarized and adapted from the original publication – presents the results of a study that assessed the potential of the non-axenic mixed algal culture of Euglena gracilis with Selenastrum sp. to produce biomass and valuable fatty acids, particularly EPA, DHA and ARA, and tocopherols, and to simultaneously purify aquaculture wastewater and improve the economy of RAS. Cultivation experiments were carried out in aquaculture wastewater both with and without amendment of sludge from the solids removal tanks of RAS systems for pikeperch (Sander lucioperca) and catfish (Clarias anguillaris).
Study setup
Aquaculture wastewaters and sludge for cultivation experiments were collected from a RAS fish pool used for growing pikeperch or catfish, and from a solids removal tank which is part of the aquaculture wastewater treatment in RAS. The volume of the RAS was 78 cubic meters (fish tank volume 65 cubic meters), and the flow rate was 35 L/sec. The RAS included a drum filter and a flotation tank for solid (later called sludge) removal, aerobic biofilters for nitrification and anaerobic biofilters for denitrification.
Before recirculation to the fish tank, the water was stripped of carbon dioxide by introducing oxygen produced on-site and dissolved into the system via an oxygen tower, and then sterilized by ozone and UV treatment. Water consumption during fish production was very low (0.05 cubic meters per kg of fish, the maximum feed load into the system was 75 kg/day, and FCRs were 1.1 to 1.2 for pike perch and 0.78 for catfish. Collected sludge enriched by drum filtering and flotation was usually delivered to municipal wastewater treatment.
The non-axenic mixed cultures of Euglena gracilis (CCAP 1224/5Z) and Selenastrum (SCCAP K-1877) were cultivated in aquaculture wastewater with and without sludge amendment. We selected the strain combination instead of a monoculture on the basis of our earlier study showing efficient wastewater treatment capacity (NH4-N uptake), better tolerance to bacterial contaminations and more efficient lipid production in a pilot scale culture of E. gracilis with the presence of Selenastrum in early cultivation phase in comparison to monoculture of E. gracilis.
Additionally, E. gracilis was chosen since it is a known producer of LC-PUFAs and has a high content of tocopherols. Cultivation media were (i) aquaculture wastewater from pikeperch pool, (ii) sludge-amended aquaculture wastewater from pikeperch pool, (iii) aquaculture wastewater from catfish pool, and (iv) sludge-amended aquaculture wastewater from catfish pool. Aquaculture wastewater without sludge-amendment were used as controls to study the influence of sludge amendment on growth, nutrient removal, and lipid and tocopherol content.
For additional information and details on the RAS unit and collection of aquaculture wastewater; microalgal cultivation; sampling and harvesting; analytical methods used; and statistical analyses carried out, please refer to the original publication.

Results and discussion
Based on the optimal N:P ratio in algal biomass, nutrient ratios in both culture wastewaters without sludge amendment were close to algal requirements (N15:P1), whereas the sludge amendment resulted in higher relative amount of P (N12:P1 in sludge-amended like perch aquaculture wastewater and N11:P1 in sludge-amended catfish aquaculture wastewater). The higher initial nutrient concentrations in the sludge-amended aquaculture wastewater enhanced the algal biomass growth.
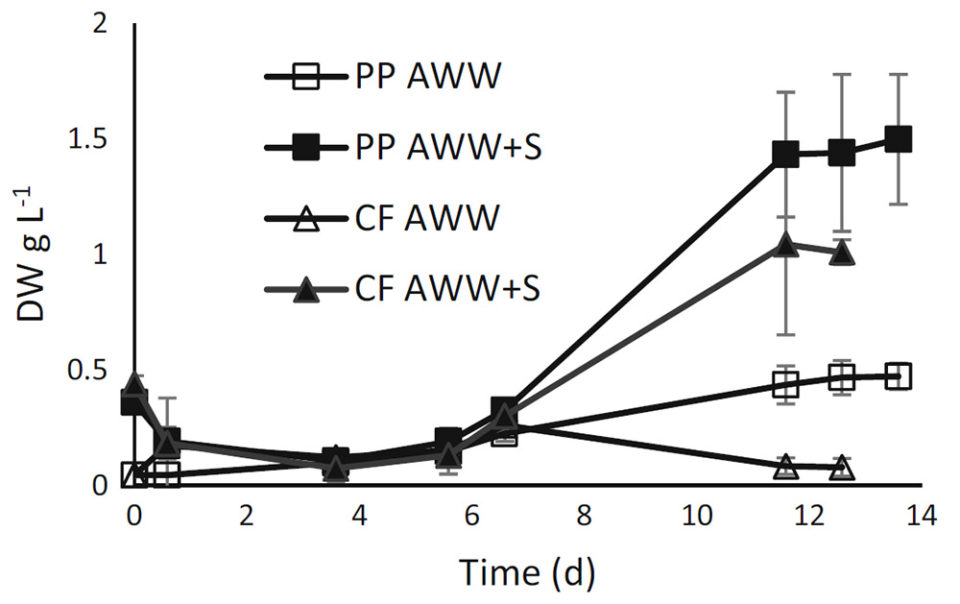
The highest biomass content (1.5 g/L) was reached in the sludge-amended pike perch aquaculture wastewater (Fig. 1) with the highest TN and TP concentrations (34.4 and 6.1 mg/L). In the sludge-amended catfish aquaculture wastewater, the algal biomass was 1.0 g/L, whereas it was only half of that (0.47 g/L) in cultures grown in pikeperch aquaculture wastewater and lowest in catfish aquaculture wastewater grown culture (0.08 g/L).
The specific growth rates (mean ± SD) during the exponential growth phase (days 1 to 7 in pikeperch aquaculture wastewater and 4 to 12 in aquaculture wastewater with sludge amendment) were 0.27 grams/day ± 0.04 in pikeperch aquaculture wastewater, 0.33 grams/day ± 0.07 in sludge-amended pike perch aquaculture wastewater, and 0.38 grams/day ± 0.21 in sludge-amended catfish aquaculture wastewater. In catfish aquaculture wastewater without sludge-amendment, the experiment resulted in negative growth rates. In all cultures, pH values were between 7.05 and 7.50 throughout cultivation.
At the end of cultivation, only E. gracilis was present in pikeperch aquaculture wastewater, and it was the strongly dominating strain also in other cultures, where the cell densities of Selenastrum at the end of the cultivation were under reliable counting limits. Bioavailable nutrients were consumed from all cultivations during the early phase of the experiment, and finally, in all cases, 98.9 to 99.5 percent and 98.4 to 99.8 percent of NH4-N and PO4-P were removed, respectively.
FA and tocopherol composition in algal biomass, total lipid, EPA and DHA contents (70.3, 7.6, and 4.5 mg/gram) were the highest in cultures grown without sludge amendment in pikeperch aquaculture wastewater. In cultures grown in catfish aquaculture wastewater without sludge amendment and in sludge-amended pike perch aquaculture wastewater, biomass lipid contents were comparable (56.5 and 55 mg/gram), and higher than in cultures grown in sludge-amended catfish aquaculture wastewater (43.6 mg/gram).
In pike perch and catfish aquaculture wastewater-grown cultures, the contents of EPA were comparable and higher (7.6 and 6.2 mg/gram) than in sludge amended pike perch and catfish aquaculture wastewater-grown cultures (4.7 and 3.7 mg/gram). The DHA content was highest in pikeperch aquaculture wastewater-grown culture (4.5 mg/gram), lowest in sludge-amended catfish aquaculture wastewater (2.3 mg/gram) and comparable in cultures grown in sludge-amended pike perch aquaculture wastewater and catfish aquaculture wastewater (3.2 and 3.3 mg/gram). The ARA content was higher in both cultures without sludge amendment (4.3 and 4.0 mg/gram in pike perch and catfish aquaculture wastewater) than in sludge-amended cultures (3.0 and 2.4 mg/gram in pike perch and catfish aquaculture wastewater).
Almost half of the total lipids in both sludge-amended algae cultures were PUFAs (48.9 and 49.2 percent in sludge-amended pike perch and catfish aquaculture wastewater, respectively), whereas in aquaculture wastewater without sludge amendment, PUFA contribution to all FAs was 56.7 percent in pike perch and 60.1 percent in catfish aquaculture wastewater. The proportions of EPA, DHA and ARA in TFAs in cultures were 8.2 to 11.2, 5.3 to 6.4, and 5.4 to 7.3 percent, respectively. Since the E. gracilis was the dominating strain in all cultures, FA composition reflected the FA composition of E. gracilis.
However, higher biomass yield in sludge-amended pikeperch aquaculture wastewater resulted in the highest lipid production (84.9 mg/L) as well as higher yields of ARA, EPA and DHA (4.6, 2.3, and 5.0 mg/L) than in other cultures, although the difference was statistically significant only for EPA. Generally, in total lipids, the proportion (percent of TFAs) of all PUFAs as well as ARA, EPA and DHA were higher and the proportion of saturated FAs (SAFAs) lower in cultures grown in aquaculture wastewater without sludge amendment than in sludge-amended cultures.
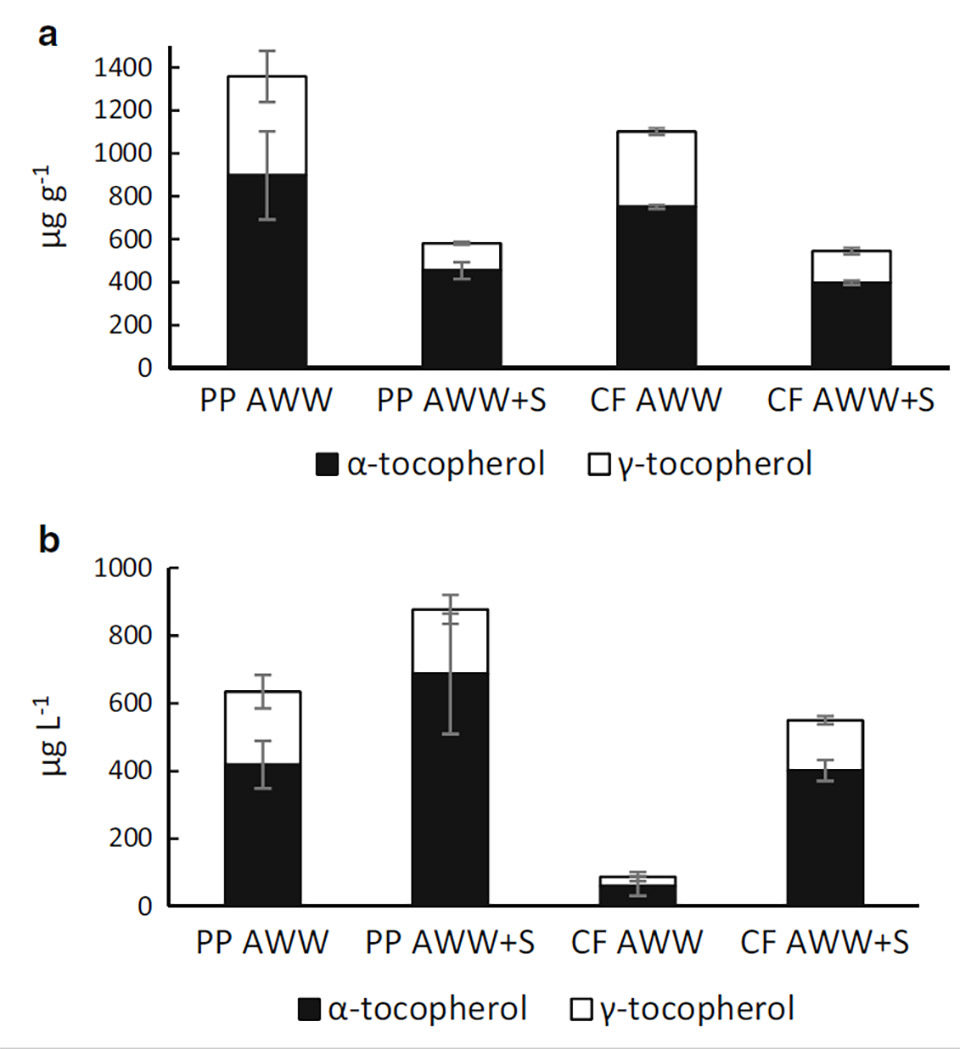
Tocopherols consisted of α- and γ-tocopherol, whereas β- and δ-tocopherols were not detected. As in the case of FAs, the total tocopherol content was higher in pike perch and catfish aquaculture wastewater without sludge amendment (1,358 and 1,102 μg/g) than in the corresponding sludge amended cultures, where the content of tocopherols was approximately 50 percent lower (580 and 545 μg/g).
The tocopherol yield was highest in the sludge-amended pike perch aquaculture wastewater grown culture, 877.2 μg/L, although there was no significant difference in comparison to cultures grown in pikeperch AWW (634.2 μg/L) or in sludge-amended catfish aquaculture wastewater (550.3 μg/L). In sludge-amended cultures in pike perch and catfish aquaculture wastewater, α-tocopherol made up 78 and 72 percent of total tocopherol, while in the cultures without sludge amendment, the proportions of α-tocopherol were lower, 66 and 68 percent.
In our algal cultures, EPA and DHA contents were close to or higher than in general required for fish feeds and exceeded the required proportions of ARA and α-tocopherol. To our knowledge, this is the first time, when RAS wastewater from pikeperch and catfish farming were used for algal cultivation and our approach to enrich wastewater with solids from sludge removal tank is unique.
The high content of LCPUFAs and tocopherols in the algal biomasses produced in our experiments indicate that aquaculture wastewater-grown mixed cultures of E. gracilis with Selenastrum are a promising substitute for at least part of the fish oil used in feed. Additionally, earlier studies have shown the high protein content of E. gracilis and green algae. However, at the end of the experiments, biomass in our cultures consisted mostly of E. gracilis biomass, and further investigations are needed to clarify the possible advantages of mixed algal cultivation in aquaculture wastewater.
Furthermore, characterization of the whole biomass composition is necessary for the evaluation of biomass suitability and safety for feed use.
Perspectives
This study showed that aquaculture wastewater from RAS units used for growing pikeperch and catfish are suitable for algal biomass production and that sludge amendment to the wastewater increases algal biomass production. Efficient reduction of nutrients and COD from aquaculture wastewater in mixed cultures of E. gracilis and Selenastrum indicates that wastewater treatment can be enhanced by integrating algal culturing units with RAS units.
EPA and DHA contents in the biomass were comparable to fish feeds containing fish and plant oils, and the ARA content exceeded the minimal requirements for feed. Tocopherol contents were superior to common plant oils.
References available from original publication.
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Author
-
Marika Tossavainen
Faculty of Biological and Environmental Sciences
Ecosystems and Environment Research Programme
University of Helsinki
Niemenkatu 73, 15140 Lahti, Finland
Tagged With
Related Posts

Responsibility
Aquaculture byproducts improve sustainability of seafood value chains
Tons of aquaculture byproducts are available as sources for fishmeal and fish oil to supplement the supplies obtained from fisheries. Innovative technologies are supporting more efficient use of these by-products in aquafeed.

Responsibility
Recovering nutrients from seafood processing water
A research project from Chalmers University of Technology, Sweden, shows the potential of recycling nutrients from seafood processing waters back into the food chain.

Aquafeeds
It takes guts to advance sustainability in aquaculture
With byproducts representing between 25 to 50 percent of the weight of various fish species, we need to be looking at how the entire fish is being used: even the heads, guts and skin.

Aquafeeds
Animal byproduct concentrates useful tools in formulation
With the market volatility of fishmeal, as well as rising sustainability concerns, the aquaculture industry is seeking sources of protein, such as animal byproduct concentrates, to substitute for fishmeal.


