Seven-week trial evaluates effects at the microcosm level

Supplemental feed and feeding are two of the most important production costs in shrimp aquaculture. They are also the main source of water quality deterioration, both in culture systems and the ecosystems that receive aquaculture effluents.
To improve the sustainability of feeding it is necessary to optimize not only the quality of feedstuffs but also their use through the most effective feeding strategies. One pending topic is the optimal utilization of natural feed (biota), which can represent up to 75 percent of the nutritional requirements of shrimp raised in ponds.
Microorganisms
According to aquaculture nutritionist Dr. Albert Tacon, it is crucial to understand the important role of natural feed, including microorganisms, in the complete nutrition of farmed shrimp. Up to the present, various zooplankton (rotifers, copepods, branquiopods and polychaete larvae) and zoobenthos (polychaetes, insects and benthic copepods) have been the most utilized as natural feeds for aquaculture.
Microorganisms have been used mostly as probiotics, rather than as direct feed biomass. However, it has become evident for many aquaculturists that with their great replication capacity, microorganisms represent an important food source. Their use during the nursery, pre-growout and growout phases of shrimp and other organisms has increased enormously in recent years.
Biofilms, bioflocs
Since shrimp are not able to consume microorganisms directly from the water column, they utilize the microbiota attached to biofilms and/or bioflocs, and floating or fixed substrates. Enriching the water column with inorganic and/or organic nutrients is another way to get microorganisms to shrimp. Diverse studies have demonstrated the nutritional value of bioflocs and biofilms, which may contain more than 30 percent protein, as well as acceptable levels of polyunsaturated fatty acids and highly unsaturated fatty acids.
Heterotrophic-based systems have been successfully operated in Brazil to nurse white and pink shrimp at densities as high as 6,000 per cubic meter, with survivals up to 90 percent. Autotrophic systems have also begun to be used in Brazil and other countries. Based mainly on diatoms and flagellates as the first link of the trophic chain, they also introduce micro- and macrosubstrates to form biofilms and/or bioflocs. An additional benefit of these systems is that the farmed organisms have higher nutritional, sanitary, immune and post-harvest quality than those nursed in traditional systems.
Mexico trial
The authors conducted a seven-week trial at the University of Sonora to evaluate the effects at the microcosm level of autotrophic- and heterotrophic-based systems on Pacific white shrimp (Litopenaeus vannamei) at the nursery and pre-grow-out phases. Plastic 120-L tanks were used for the first phase, and 1,200-L tanks were used for the second. A traditional nursery system functioned as a control. No artemia was used in any of the treatments.
The stocking density for the nursery phase was 900 postlarvae (P.L.) per tank. After three weeks, surviving juveniles were transferred to the pre-grow-out units and harvested after four additional weeks. Constant aeration was supplied in all the experimental units. From day 5 until the end of the trial, shrimp received a formulated feed at a rate of 8 percent of their total biomass.
All the experimental units were provided with wheat bran (10 and 100 grams per tank for nursery and pre-grow-out phases, respectively) and plastic mesh (0.15 and 0.6 square meters per tank for the two phases) for the formation of bioflocs and biofilms. For the autotrophic system, an agricultural fertilizer was supplied to maintain a nitrogen:phosphorus (N:P) ratio around 8:1. The tanks were put outdoors and exposed continuously to sunlight.
For the heterotrophic system, the water column was additionally fertilized with molasses, and the N:P ratio was maintained over 20:1. The tanks were put under a metallic structure and covered with plastic shades to minimize the penetration of sunlight.
Experimental results
Table 1 shows the production responses of shrimp in the two systems and the control. The best final weights and biomasses for the complete nursery/pre-grow-out trial were recorded in the control and the heterotrophic system. The best survival in both phases was found in the autotrophic system. The feed-conversion ratios during the nursery and pre-grow-out phases were similar and very low in all treatments.
Cordova, Mean values for production variables, Table 1
| Initial Weight (mg) | Final Weight (mg) | Final Biomass (g/m3 | Survival Nursery (%) | Survival Pre-Growout (%) | Feed Conversion Nursery | Feed Conversion Pre-Growout |
|---|
Initial Weight (mg) | Final Weight (mg) | Final Biomass (g/m3 | Survival Nursery (%) | Survival Pre-Growout (%) | Feed Conversion Nursery | Feed Conversion Pre-Growout | |
|---|---|---|---|---|---|---|---|
| Control | 15 ± 2a | 1,819 ± 86a | 5,142 ± 273.32 | 74.2 ± 3.2b | 42.1 ± 2.4b | 0.69 ± 0.09a | 0.58 ± 0.09a |
| Autotrophic | 15 ± 1a | 1,137± 81b | 4,044 ± 252.2a | 84.6 ± 4.5a | 70.7 ± 4.1a | 0.67 ± 0.06a | 0.54 ± 0.06a |
| Heterotrophic | 16 ± 2a | 1,214 ± 76a | 4,429 ± 256a | 74.6 ± 3.5b | 48.3 ± 3.1b | 0.65 ± 0.05ad | 0.55 ± 0.05a |
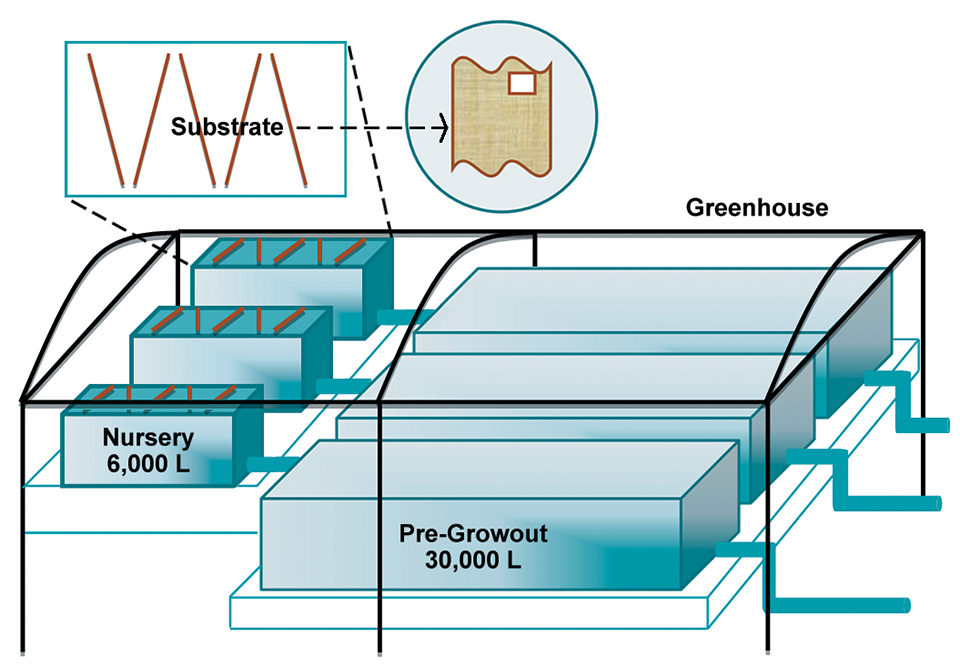
Based on the good results of the experimental phase, it is feasible to implement this project at a pilot or commercial level (Fig. 1). The idea is to further evaluate the production responses of shrimp, as well as their nutritional, sanitary and immune traits, and post-harvest quality.
(Editor’s Note: This article was originally published in the March/April 2011 print edition of the Global Aquaculture Advocate.)
Authors
-
Dr. Luis Rafael Martinez-Cordova
Departamento de Investigaciones Científicas y Tecnológicas de la Universidad de Sonora
Blvd. L. Donaldo Colosio s/n
Entre Sahuaripa y Reforma, Edifico 7G
Hermosillo, Sonora 83000 Mexico -
Dr. Manuel de Jesus Becerra-Dorame
Departamento de Investigaciones Científicas y Tecnológicas de la Universidad de Sonora
Blvd. L. Donaldo Colosio s/n
Entre Sahuaripa y Reforma, Edifico 7G
Hermosillo, Sonora 83000 Mexico -
Dr. Marcel Martinez-Porchas
Centro de Investigación en Alimentación y Desarrollo
Hermosillo, Sonora, Mexico
Tagged With
Related Posts

Health & Welfare
A holistic management approach to EMS
Early Mortality Syndrome has devastated farmed shrimp in Asia and Latin America. With better understanding of the pathogen and the development and improvement of novel strategies, shrimp farmers are now able to better manage the disease.

Health & Welfare
A study of Zoea-2 Syndrome in hatcheries in India, part 1
Indian shrimp hatcheries have experienced larval mortality in the zoea-2 stage, with molt deterioration and resulting in heavy mortality. Authors investigated the problem holistically.

Health & Welfare
Ammonia addition enhances microbial flocs in nursery phase for Pacific white shrimp
In a study, “pre-fertilization” in the nursery phase of a biofloc system for shrimp was tested. The objective was to accelerate the biofloc formation to minimize ammonia concentrations, avoiding high peaks during culture.

Health & Welfare
Biofloc technology holds potential for carnivorous fish species
Juvenile carnivorous African catfish performed well in biofloc-based systems, which could help produce better quality and more disease-resistant seed of this important aquaculture species and support the expansion of African catfish farming industry.


