Kentucky State University builds baseline genetic assessment of available strains

The Malaysian prawn (Macrobrachium rosenbergii), also known as the giant freshwater prawn or giant river prawn, is a species of significant global importance with an annual global production of over 200,000 metric tons (MT). However, cultured stocks of freshwater prawns in the Western Hemisphere are primarily, if not entirely, derived from only 36 individuals introduced to Hawaii from Malaysia during the mid-1960s. Since then there has been no documented addition of new broodstock.
Prawn stocks in this region have thus experienced long-term culture without the introduction of new genetic resources. This makes it likely that “founder effects” were compounded as new cultured populations were established. These sequential bottlenecks are known to potentially reduce levels of genetic diversity. Also, populations established from a limited number of individuals have a higher likelihood of inbreeding, which can negatively affect stock productivity via inbreeding depression.
In an effort to determine if there is evidence of genetic deterioration in cultured prawn stocks and potentially establish a freshwater prawn genetic-improvement program, a series of preliminary studies were conducted at Kentucky State University in Frankfort, Kentucky, USA. The goals of these studies were to provide a baseline genetic assessment of some available strains and compare the hatchery and grow-out performance of some commercially available strains.
Genetic diversity
As genetic diversity is the fundamental resource on which stock improvement programs rely, it is important to first develop an understanding of available genetic variability. To evaluate genetic diversity, tissue samples were collected from seven cultured and two wild prawn populations and diversity was estimated at five microsatellite loci. Cultured populations included Hawaii-1, Hawaii-2, India-cultured, Israel, Kentucky, Mississippi and Texas, and the wild populations included Myanmar and India-wild.
These analyses found that the wild Myanmar and cultured India populations possessed significantly higher estimates of allelic richness and expected heterozygosity than all other populations (Fig. 1). Allelic richness was significantly higher in the Hawaii populations than the Kentucky, Texas, Mississippi, India-wild and Israel populations.
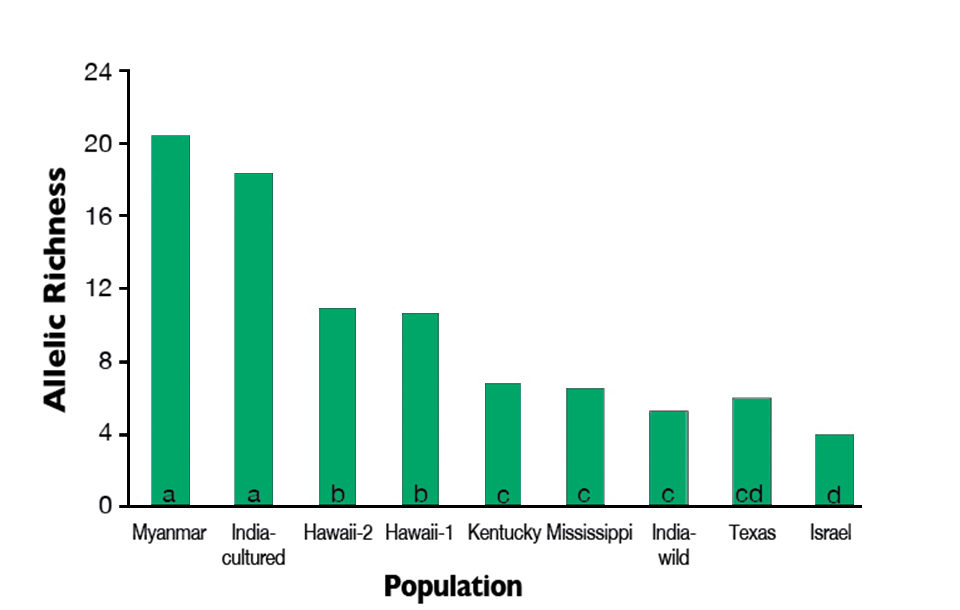
(P ≤ 0.05).
The allelic richness values of the Kentucky, Texas, Mississippi and India-wild populations were not significantly different from each other. However, the allelic richness of the Israel population was significantly lower than in all other populations except Texas.
Expected heterozygosity was not significantly different among the Hawaii-1, Hawaii-2, Kentucky, Texas, Mississippi and India-wild populations. Expected heterozygosity in the Israel population was significantly lower than the Hawaii-1, Hawaii-2 and Mississippi populations (Fig. 2).
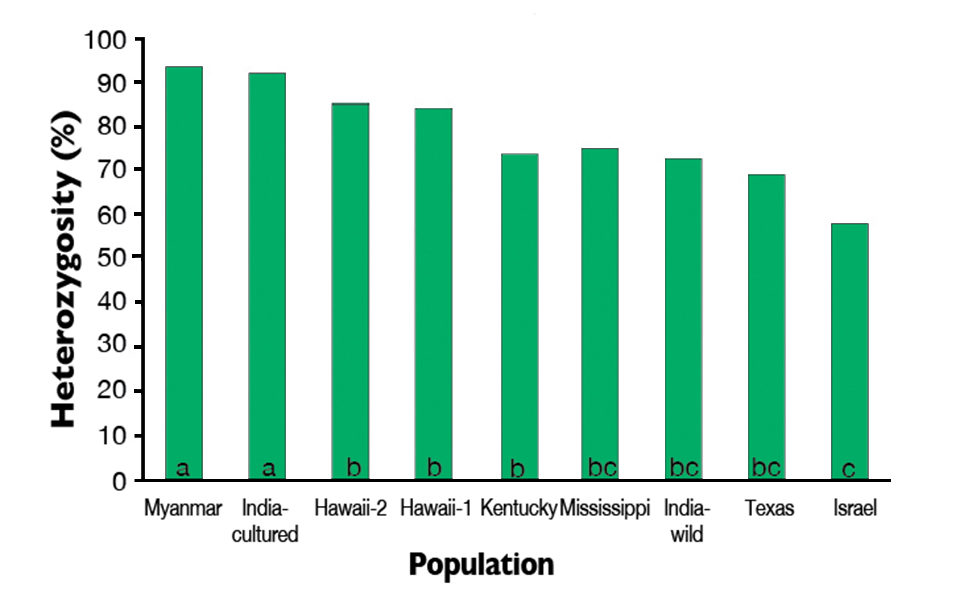
Genetic differentiation among populations was moderately high with an overall fixation index (Fst) value of 0.1569. Multidimensional scaling of estimated pairwise Fst values revealed two separate population groups. The first group included the two Hawaii populations and the Myanmar and India-cultured populations. The second group consisted of the Kentucky, Mississippi and Texas populations with the Kentucky and Mississippi populations grouping closely.
Of the populations evaluated in the study, the Myanmar and India-cultured populations appeared to be the greatest resources of genetic diversity for stock improvements. Secondarily, the Hawaii populations displayed moderately high levels of within-population diversity. The India-wild, Kentucky, Mississippi, Texas and Israel populations appeared to be relatively poor resources of genetic diversity, as they exhibited substantial reductions of within-population diversity.
Based on these results, broodstock were procured from the Texas, Hawaii and Myanmar populations. The rationale for selecting these populations for further evaluation was based on the Texas population being the most widely used strain in the continental United States (serving as the reference strain), the Myanmar population exhibiting the greatest diversity and the Hawaii population being a readily available commercial strain that exhibits moderate differentiation from the Texas strain.
Hatchery trial
An initial trial conducted in the spring of 2009 compared the hatchery performance of the three strains. Since the costs of juveniles is over 50 percent of operational cost for most prawn producers, improvements in the hatchery, such as increased fecundity, increased survival or a decreased number of days to achieve metamorphosis to postlarvae, could result in significant savings in hatchery operations.
Gravid females from all three strains were collected from a broodstock holding system and placed into three 700-L larvae collectors and acclimated to 12 ppt salinity. Larvae were hatched over a 48-hour period, then volumetrically counted and stocked into 12, 266-L rearing tanks at a density of 60 larvae/L with four replicate tanks for each strain. Larvae were fed according to a feed chart. Gravid females were also collected for egg clutch removal to determine fecundity and hatch success.
The results of this trial indicated that at 32 days, the larvae from the Texas strain required two fewer days to complete metamorphosis than the Hawaii and Myanmar strains. Survival to postlarvae was significantly higher in the Texas and Hawaii strains compared to the Myanmar strain (Fig. 3). Fecundity was highest in the Texas strain. Hatch success was significantly higher in the Myanmar strain than in the Texas strain, with the Hawaii strain performing intermediately.
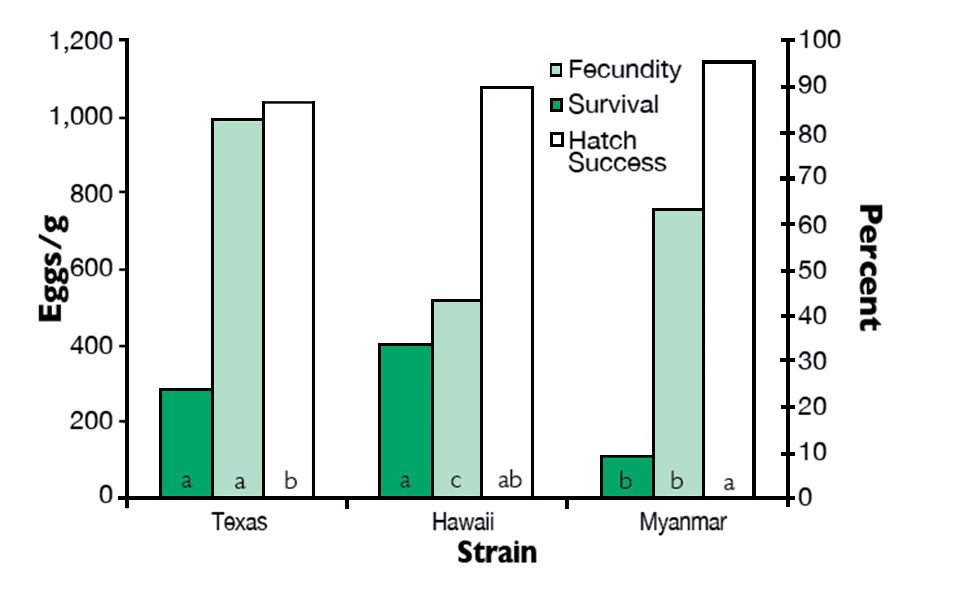
of M. rosenbergii. Lower-case letters in bars denote significant differences (P ≤ 0.05).
Grow-out trial
A subsequent pond grow-out trial was conducted in the summer of 2009 to evaluate the performance of the three pure strains under low and high densities. Forty-five-day nursed juvenile prawns (0.8 ± 0.3 grams) from the three strains were stocked into 18, 0.04-hectare (ha) ponds at either 24,700/ha (low density) or 74,000/ha (high density with added substrate).
Prawns were fed 28 percent protein sinking pellets once daily at a standardized rate. Dissolved oxygen, temperature and pH were monitored twice daily, and total ammonia nitrogen, nitrite, alkalinity and hardness were monitored twice weekly. At the end of a 16-week period, all prawns were harvested, bulk weighed and counted.
Under both management strategies, the commercial Hawaii and Texas strains performed very similarly and better than the wild Myanmar strain in terms of total production and average weight (Table 1). Survival was significantly higher in the Texas and Hawaii strains when compared to the Myanmar strain under high-density management. However, under low density, survival of the Texas strain was higher than the Myanmar strain, while the survival of the Hawaii strain was not significantly different from that of the others.
Schneider, Production parameters for three genetic strains, Table 1
| Variable | Low-Density Culture Texas | Low-Density Culture Hawaii | Low-Density Culture Myanmar |
|---|
Variable | Low-Density Culture Texas | Low-Density Culture Hawaii | Low-Density Culture Myanmar |
|---|---|---|---|
| Total production (kg/ha) | 986.70 ± 40.60a | 826.50 ± 82.22a | 468.80 ± 49.34b |
| Average harvest weight (g) | 41.79 ± 1.22a | 38.76 ± 2.80a | 25.27 ± 4.00b |
| Survival (%) | 95.30 ± 1.10a | 85.77 ± 3.68ab | 76.87 ± 5.23b |
| High-Density Culture | High-Density Culture | High-Density Culture | |
| Total production (kg/ha) | 2,585.20 ± 90.73a | 2,280.20 ± 142.96a | 1,409.60 ± 136.20b |
| Average harvest weight (g) | 36.82 ± 1.19a | 33.70 ± 2.45a | 23.83 ± 2.03b |
| Survival (%) | 94.67 ± 0.68a | 91.22 ± 1.12a | 79.91 ± 2.22b |
Lower-case letters denote significant differences (P ≤ 0.05).
These results likely demonstrated the positive impacts of the process of domestication. Inbreeding depression in the commercial strains was not indicated by these data.
(Editor’s Note: This article was originally published in the November/December 2010 print edition of the Global Aquaculture Advocate.)
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Authors
-
Kyle J. Schneider
Aquaculture Research Center
Kentucky State University
Frankfort, Kentucky 40601 USA -
Oluwapelumi Odeyemi
Aquaculture Research Center
Kentucky State University
Frankfort, Kentucky 40601 USA -
David R. Wood
Aquaculture Research Center
Kentucky State University
Frankfort, Kentucky 40601 USA -
Shawn D. Coyle
Aquaculture Research Center
Kentucky State University
Frankfort, Kentucky 40601 USA -
Boris Gomelsky
Aquaculture Research Center
Kentucky State University
Frankfort, Kentucky 40601 USA -
James H. Tidwell, Ph.D.
Aquaculture Research Center
Kentucky State University
Frankfort, Kentucky 40601 USA
Tagged With
Related Posts

Intelligence
Culture of giant freshwater prawns in China
Farming of giant freshwater prawns is very popular in China. The Yangtze River Delta region produces more than 60 percent of the country's output. Production increases have resulted from a novel system that involves greenhouses that allow ponds to be stocked ahead by two months.

Aquafeeds
Feeding strategy supports freshwater prawns without fishmeal, fish oil
Low-input culture practices for freshwater prawns can manage their growth and biological characteristics so they can be fed no fishmeal or fish oil.

Health & Welfare
Examining domestication of green tiger shrimp in Egypt
The domestication of green tiger shrimp (Penaeus semisulcatus) as a source for seedstock could help expand aquaculture in Egypt. Trials by the authors compared the reproductive performance of wild male/wild female pairings with that of pond-reared male/pond-reared female and wild male/pond-reared female pairings.

Health & Welfare
Biosecurity principles for sustainable production using SPF shrimp
Basic components of biosecurity include knowledge of diseases, adequate detection methods and the use of “clean” shrimp stocks.


