Culture in large quantities adds complexity for small-mouthed species

The culture of many marine fish species requires the concurrent culture of multiple species of live feed such as algae and microcrustaceans. Larval marine fish require at least artemia nauplii, usually enriched, and some require both enriched artemia and enriched rotifers. The most difficult to culture marine fish species require copepods, either due to the size needed for the small mouth size of the fish or the superior nutritional quality of copepods compared to rotifers.
Many species of marine fish with smaller mouths exhibit extremely high mortality rates during the first feeding period two weeks post-hatch or have proven impossible to culture. The addition of copepods to first feeding often improves survival, but the culture of copepods in large quantities adds another level of complexity to the culture protocols for small-mouthed species.
Copepod research at GCRL
The Gulf Coast Research Laboratory (GCRL) in Ocean Springs, Miss., USA, has been harvesting wild copepods and/or culturing captive strains for the last decade. The facility has investigated many ways to increase the scale of production and simplify the labor required to culture copepods. In addition, the transmission of pathogens through the cultured food chain has been a concern.
Researchers at GCRL designed a bio-secure indoor production system for the marine copepod Acartia tonsa that combines batch culture of adults with the continuous culture of eggs, maximizing production and minimizing labor. A. tonsa was chosen because it is a local species, and the size ranges of nauplii, copepodites and adults are appropriate for many marine fish that require copepods for first feeding.
The copepods produced at GCRL have been used almost exclusively for the culture of Gulf of Mexico red snapper (Lutjanus campechanus). Red snapper is an overfished species in the gulf, and its harvest is restricted for both recreational and commercial fishing.
The GCRL project is focused on developing the technology to culture red snapper through its life cycle in order to restock public waters and create a commercial aquaculture industry. GCRL has produced and restocked thousands of red snapper juveniles onto reefs off the coast of Mississippi. Mortality in the hatchery phase has been high.
Biosecure system
The GCRL copepod systems evolved from a wild-harvest, 80-metric ton outdoor tank system to a greenhouse-enclosed, two-phase batch tank system to the current bio-secure indoor batch/continuous system. The indoor system was built in a climate-controlled building that receives ultraviolet-filtered air. The room and all tanks were sanitized before operation using chlorine.
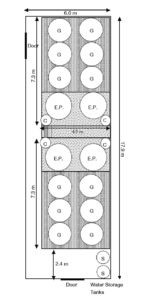
The bio-secure system is composed of four separate but identical subsystems (Fig. 1). Each subsystem has three circular 900-L cone-bottom grow-out tanks linked together by 4-cm pipe that empty by gravity into a single 2,200-L cone-bottom egg production tank. Water and eggs are withdrawn by gravity through a 200-µ screen on a 10-cm center stand pipe that flows into a 50-µ plankton net suspended in a 200-L egg collection tank.
Water returns from the bottom of the egg collection tank to the egg production tank via a 4-cm airlift with a flow of 10-14 L/minute. Each tank receives a gentle supply of air through 4-cm-square air stones. This aeration helps to evenly distribute the algae and copepods. A wooden platform surrounds the tanks to provide a working surface for the staff.
Production sequence
The first step in the production sequence is the stocking of the grow-out tanks with freshly harvested copepod eggs. The tanks receive feed daily beginning with 0.5 L (approximately 25,000 cells/mL) of Isochrysis galbana algae and increasing to 5 L (approximately 200,000 cells/mL) of algae on day 7.
The second step occurs every five days, when half the volume of one of the egg production tanks is drained, and the entire volume of a 14-day-old grow-out tank is added. The egg production tanks receive 11 L (200,000 cells/mL) of algae every day.
The third step is the harvest of the egg collection tanks each day. The plankton nets in all the tanks are sprayed down, the cod-end harvested, and all eggs transferred to a beaker for counting.
A portion of the eggs is used to start another grow-out tank, and the remainder is hatched out to feed the young red snapper larvae. The eggs are then incubated in circular 200-L egg-hatching tanks. After hatching for 12 hours, the nauplii are added to the snapper larval-rearing tanks. The average daily harvest of eggs ranges from 6 million to 16 million eggs over a six-month period. After hatching, these eggs produce a range of 4 million to 6 million nauplii.
All tanks are filled with reverse-osmosis-filtered, artificial seawater. The seawater is mixed with commercially available salt and tap water. The temperature averages 25 degrees-C, and the salinity is maintained at 25 ppt. All nitrogen waste products remain at low levels due to the algae, and all system water is reused in GCRL fish production systems
(Editor’s Note: This article was originally published in the July/October 2013 print edition of the Global Aquaculture Advocate.)
Authors
-
Brie Sarkisian
Thad Cochran Marine Aquaculture Center
Gulf Coast Research Laboratory
Ocean Springs, Mississippi 39564 USA[117,100,101,46,109,115,117,64,110,97,105,115,105,107,114,97,115,46,101,105,114,98]
-
Jason Lemus
Thad Cochran Marine Aquaculture Center
Gulf Coast Research Laboratory
Ocean Springs, Mississippi 39564 USA -
Phillip Lee
Thad Cochran Marine Aquaculture Center
Gulf Coast Research Laboratory
Ocean Springs, Mississippi 39564 USA
Tagged With
Related Posts

Health & Welfare
Advances in intensive copepod production technology
Research at the Oceanic Institute has been successful in overcoming bottlenecks associated with rearing small-mouthed fish larvae by finding a suitable first feed. Early work on the calanoid copepod Parvocalanus crassirostris focused on parameters necessary for successful maintenance of stock cultures.

Health & Welfare
Aquamimicry: A revolutionary concept for shrimp farming
Aquamimicry simulates natural, estuarine production conditions by creating zooplankton blooms as supplemental nutrition to the cultured shrimp, and beneficial bacteria to maintain water quality. Better-quality shrimp can be produced at lower cost and in a more sustainable manner.

Responsibility
Assessing culture potential of red emperor snapper in New Caledonia
The red emperor snapper, known as “pouatte” in New Caledonia, is valuable throughout its broad geographic range and a highly valued food fish locally. Declining wild catches and market demand have provided the incentive to carry out technical feasibility studies to determine its commercial aquaculture potential.

Intelligence
Bahamas venture focuses on grouper, other high-value marine fish
A new venture under development in the Bahamas will capitalize on Tropic Seafood’s established logistics and infrastructure to diversify its operations from processing and selling wild fisheries products to include the culture of grouper and other marine fish.


