Evaluation trials could help feed manufacturers formulate improved artificial diets
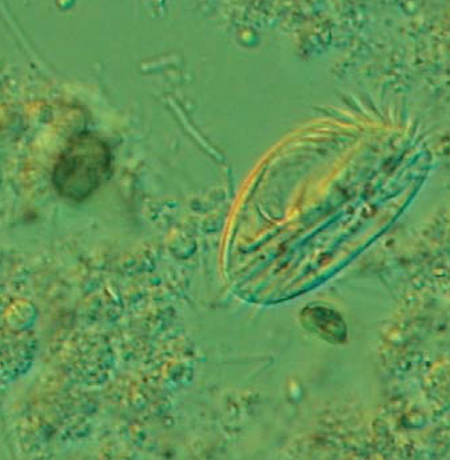
Recent work on alternative live foods for fish larvae suggests that some ciliated protozoa (ciliates) may be a valuable complimentary source of nutrients for first feeding larval stages. Traditionally, rotifers have been used as the initial food for marine fish larvae. However, for some species of fish, such as groupers and surgeonfishes, the mouth opening of the larvae is too small to allow them to feed on rotifers. Alternative, smaller-sized prey such as ciliates are thus needed for larviculture of these economically important species.
Ciliates
Ciliates are eukaryotic, unicellular organisms that are abundant in most aquatic environments, including oceanic waters, wastewater, and aquaculture ponds where concentrations of 104 cells per mililieter have been reported. Generally, ciliates are categorized as loricate and aloricate, and they have a doubling time of 1 to 2 per day. Recently, Nagano et al. (Plankton Biol. Ecol. 47:93-99; Hydrobiologia 432:149-157) demonstrated that high concentrations of ciliates enhanced larval fish survival until 4-6 days post hatching, without the addition of rotifers (Table 1). These results indicate that ciliates can play an important role as an alternative food source by enhancing larval fish survival (especially for those species with a small mouth), and that ciliates may bridge the gap until the larvae attain a size when they can feed on rotifers.
Enhanced nutrition
Additionally, ciliates may provide nutrients that are not present, or present at low concentrations, in other live foods.
Decamp, Survival of fish larvae reared with ciliates, Table 1
| Fish Species | Ciliate Density (no./ml) | Survival (%) |
|---|---|---|
| Grouper, Epinephelus septemfasciatus | ||
| With naked ciliates (Euplotes sp. 1) | 12 | 38 ± 10 |
| Without ciliates | 0 | 16 ± 7 |
| Grouper, E. septemfasciatus | ||
| With loricate ciliates (Favella taraikaensis) | 5 | 24 ± 1 |
| Without ciliates | 0 | 1 ± 1 |
| Surgeonfish, Paracanthurus hepatus | ||
| With loricate ciliates (Amphorellopsis acuta) | 10 | 48 ± 4 |
| With aloricate ciliates (Euplotes sp. 2) | 13 | 12 ± 1 |
| Without ciliates | 0 | 0 |
HUFAs and sterols
Protozoa such as ciliates and flagellates are sources of highly unsaturated fatty acids and/or sterols that have a known growth-promoting effect on most invertebrates and fish larvae. The neutral lipids of a species of scuticociliate were reported to consist of 29 percent sterols, 53 percent of which were cholesterol.
Trophic status important
However, the biochemical composition of ciliates will change with the trophic status of the species. For example, bacterivorous ciliates (C:N ratio of 6.2 reported for a scuticociliate) are expected to be a poorer source of nitrogen than algivorous ciliates (C:N ratio of 4.6 to 4.7 reported for tintinnids). Similarly, the fatty acid composition of bacterivorous ciliates often includes substantial quantities of bacterial fatty acids.
Free amino acids
Finally, marine ciliates characteristically contain high intracellular concentrations of free amino acids that may be an important source of energy for larvae, especially for first feeding larval fish during the critical period after yolk-sac absorption.
Common feeding protocols
Although artificial feeds are increasingly being used in penaeid shrimp larviculture, the addition of known quantities of both live microalgae (such as Chaetoceros) and artemia to the larval rearing tanks is still the most common method to feed zoea and mysis stages of shrimp. A common protocol consists of providing microalgae (5 to 20 µm diameter) at the nauplius-5 or zoea-1 stage (1 to 2 days post hatching) and feeding artemia at the mysis-1 stage (six days post hatching).
Alternative live prey
However, because of growing concerns about the availability and cost of artemia, the use of alternative live prey has been investigated. For example, a mixture of nematodes and algae were reported to outperform the traditional artemia and algae diet. Also, larvae of the Pacific white shrimp (Litopenaeus vannamei) were reared solely on nematodes and rotifers, without the addition of microalgae. However, these larvae had lower survival and growth compared with microalgae-fed controls.
Interestingly, alternative live prey, such as ciliates that are characterized by a intermediate size, have not been adequately evaluated as an alternative live food for shrimp larvae, although Thompson et al. (1999) showed that larvae of Penaeus paulensis grew better when fed flagellates and ciliates compared with a diet exclusively of bacteria.
Conclusion
We propose that evaluating alternative live foods – such as ciliates – might be of value for two reasons. Firstly, the availability of alternative live foods to supplement or replace artemia would provide hatchery managers with greater options than what currently available using traditional methods of larval feeding. Secondly, and perhaps more importantly, information collected during evaluation trials using alternative live foods could be used by the feed manufacturing industry to formulate improved artificial diets. Artificial diets currently are formulated based on the biochemical composition of traditional live foods. By evaluating alternative live foods, we may be able to test species-specific nutrients that may positively impact larval shrimp and fish performance.
(Editor’s Note: This article was originally published in the October 2001 print edition of the Global Aquaculture Advocate.)
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Authors
-
Olivier Decamp, Ph.D.
The Oceanic Institute
41-202 Kalanianaole Highway
Waimanalo, Hawaii 96795 USA -
Shaun Moss, Ph.D.
The Oceanic Institute
41-202 Kalanianaole Highway
Waimanalo, Hawaii 96795 USA -
Naoki Nagano, Ph.D.
Division of Fisheries Sciences
Miyazaki University
Miyazaki, Japan
Related Posts

Health & Welfare
A study of Zoea-2 Syndrome in hatcheries in India, part 1
Indian shrimp hatcheries have experienced larval mortality in the zoea-2 stage, with molt deterioration and resulting in heavy mortality. Authors investigated the problem holistically.

Health & Welfare
Asepsis key to prevent contamination in shrimp hatcheries
Maintaining biosecurity and asepsis in larval shrimp production is a key component of the production chain in Ecuador, which requires the production of 5.5 billion larvae monthly from 300-plus hatcheries.

Aquafeeds
Live diets for larval fish, shrimp: Production, enrichment, feeding strategies
Live diets for reared marine larvae must be cost-effective and versatile while providing good nutrition and being easily captured and digested. Copepods offer superior nutritional value, but their rearing requires space and is laborious.

Health & Welfare
Microbial flocs for aquaculture
Microbial flocs consist of a variety of bacteria, fungi, microalgae and other organisms suspended with detritus in culture water.


