A look at aeration, oxygenation

While ammonia-nitrogen build-up can severely reduce a recirculating system’s carrying capacity, the maintenance of adequate dissolved oxygen (D.O.) concentrations in the culture tank and biofilter is also of primary importance. In many situations, the capacity for adding oxygen to water becomes the first limiting factor in a system’s carrying capacity.
To maintain adequate D.O. levels in culture tanks, dissolved oxygen must be added at a rate equal to that of the rate of consumption by fish and bacteria, and whatever oxygen flows out at the tank drains. The uptake rate of dissolved oxygen in a recirculating system is difficult to calculate, yet an estimate is essential for proper system design.
The overall rate of oxygen used in a system is the sum of the respiration rate of the fish, the oxygen demand of bacteria breaking down organic wastes and uneaten food, and the oxygen demand of nitrifying bacteria in the biofilter. The amount of oxygen required is largely dictated by the feed rate and the length of time waste solids remain within the culture and water filtration system.
In systems with non-submerged biofilters and where solids are removed quickly from the tank and filtration system, as little as 0.3 kg of oxygen can be consumed for every 1.0 kg of feed added. In systems with submerged biological filters and where waste solids are retained between back-washings of filters, as much as 1.0 kg of oxygen is consumed for every 1.0 kg of added feed.
In a typical intensively loaded recirculating system, aeration or oxygenation system failure can lead to a total loss of the fish crop in 30 minutes or less unless backup oxygen or aeration is provided to the system. The term aeration is used here to refer to the dissolution of oxygen from the atmosphere into water. The transfer of pure oxygen gas into water is known as oxygenation.
Aeration
The addition of oxygen from the atmosphere into water can be accomplished in recirculating systems through a variety of devices, such as air diffusers, surface agitators and non-pressurized packed columns. System aeration generally is carried out within culture tanks, although this is not always the best place to add dissolved oxygen.
The reason is twofold. Vigorous aeration in culture tanks resuspends settled waste solids and can break them into smaller particles that are more difficult to remove from water. Also, the oxygen transfer efficiency of aerators drops as the concentration of dissolved oxygen increases to near saturation levels. However, culture tanks remain the most common place to aerate water.
Diffused aeration
Adding oxygen to a recirculating system by aerating only the water flowing back to the culture tanks does not usually supply an adequate amount of oxygen for fish production. The oxygen volume is limited by the flow rate and the generally low concentration of dissolved oxygen in water. Therefore, most aeration in recirculating systems occurs in the culture tanks. The most efficient aeration devices are those that move water into contact with the atmosphere, such as paddlewheels, propeller aspirators and vertical lift pumps. However, these methods generally create too much turbulence within a culture tank to be widely used.
The most common way to aerate in tank systems is therefore with diffused bubble aeration. These systems provide low-pressure air from a “regenerative” blower to diffusers placed on the bottoms of the culture tanks. The diffusers produce small air bubbles that rise through the water column and transfer oxygen from the bubbles to the water.
Studies have determined that diffused aeration systems can transfer oxygen at an average rate of 1.3 kg oxygen/kWh under standard test conditions with clean water at 20 degrees C. However, this value must be corrected to approximate actual fish culture conditions.
To achieve acceptable fish growth rates, depending on the species, D.O. concentration should be maintained at 5 mg oxygen/L or higher. At this concentration and a water temperature of 28 degrees C, the diffuser system’s oxygen transfer rate is only 35 percent of the estimated rate under standard conditions. In this case, the oxygen transfer rate would be reduced to 0.46 kg/kWh.
In a well-designed recirculating system with quick removal of solids, the oxygen consumption rate can be estimated as 50 percent of the feed rate. In a system that receives 45 kg of feed over an 18-hour period, the estimated oxygen consumption rate would be approximately 1.25 kg oxygen/hour.
With an actual oxygen transfer efficiency noted above, a diffused aeration system would require a blower of approximately 2.75 kW output to provide an adequate amount of dissolved oxygen. If the fish are going to be fed over a shorter period of time, then peak oxygen demand should be estimated, and the blower and diffuser capacity should be increased. The density of fish production with aeration alone is typically limited to 30-40 kg fish per cubic meter of culture tank volume.

Oxygenation
In intensive tank-based aquaculture production systems with fish densities above 40 kg/m3, the rate of oxygen consumption by the fish and bacteria usually exceeds the capabilities of typical aeration equipment to dissolve atmospheric oxygen into the water. In these cases, gaseous oxygen is used to increase the rate of oxygen diffusion to satisfy a higher oxygen utilization rate.
The saturation concentration of atmospheric oxygen in water rarely exceeds 8.75 mg/L in water with temperatures above 20 degrees-C. When pure oxygen gas is used, the saturation concentration of oxygen in water is increased nearly fivefold at standard atmospheric pressures. This allows a more rapid transfer of oxygen into water, even when the tank’s ambient dissolved-oxygen concentration is maintained close to atmospheric saturation.
A measure of success in using pure oxygen in aquaculture is the oxygen-absorption efficiency of the equipment used. Absorption efficiency is defined as the ratio of the weight of oxygen absorbed by the water to the weight of oxygen applied through the diffusion or injection system.
Properly designed oxygen-diffusion devices can produce an oxygen-absorption efficiency of more than 90 percent. However, as with aeration with air, culture tanks are not the best location for oxygen diffusion with common “air stone” diffusers. Because of the short contact time for bubbles rising through shallow water columns of less than 2 m in tanks, even flat-plate diffusers have oxygen-absorption efficiencies no greater than 40 percent.
Efficient oxygen-injection systems are designed to maximize the oxygen/water contact area and time. This can be achieved with a component like a down-flow bubble contactor, a pressurized packed-column or a low-head oxygenator.
Sources of oxygen gas include compressed oxygen cylinders, liquid oxygen and on-site oxygen generators. In most applications, the choice is between bulk liquid oxygen and an oxygen generator. The selection of the source should consider the cost of bulk liquid oxygen, which is usually dependent on distance from the oxygen production plant, and the reliability of the electrical service needed for generating oxygen on site.
Down-flow bubble contactors
A properly designed low- to medium-pressure oxygen contact system can transfer more than 90 percent of the oxygen injected through the component. One such system is a down-flow bubble contactor (DFBC), also called an oxygen cone in some designs. A DFBC system consists of a cone-shaped reactor with a water and oxygen input port at the top.
As water and oxygen bubbles move down the cone, the flow velocity decreases until it equals the upward velocity of the bubbles. This allows a long contact time between the water and oxygen bubbles and nearly 100 percent absorption of the injected gas. DFBCs also incorporate pipes and fittings of various sizes. The top sections are of smaller diameter, and the pipe diameter increase in three steps moving downward. The dissolved-oxygen concentration of water leaving a DFBC can be as high as 25 mg/L, given a system pressure of approximately 1 bar (14.7 psi).
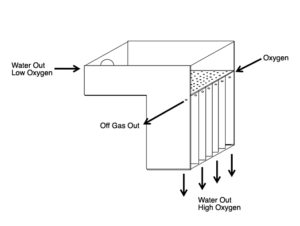
Low-head oxygenation system Multistage low-head oxygenators oxygenate flowing water where there is only a small elevation difference between the source of the water and the culture tank. This situation is often found in raceway systems set up in series and “stepped down” a sloped property. In these systems, the outflow of one raceway may be just 0.2 to 1.0 m above the inflow of an adjacent raceway.
Low-head oxygenators are made up of a perforated, horizontal distribution plate and multiple, adjacent vertical contact chambers. Gaseous oxygen enters one contact chamber, and oxygen with off gases nitrogen and carbon dioxide exits to the adjacent contact chamber. The oxygen transfer capability of these systems is determined by the length of water fall, gas and water flow rates, the D.O. concentration of the influent water and the number of contact chambers.
Including packing medium in the contact chambers can improve performance. However, the media can become clogged if the system water has a moderate concentration of suspended solids or dissolved organic solids.
Pressurized packed columns
Pressurized packed columns are usually operated in a partially flooded mode. That is, water fills part of the reactor. Water enters the top of a pressurized vessel that contains a media with a high specific surface area. Oxygen gas is introduced at the top or bottom of the column and travels upward, counter to the water flow.
As these reactors can be operated at high pressures of 3 to 4 bar (40 to 60 psi), oxygen transfer efficiency can exceed 90 percent with effluent dissolved-oxygen concentrations in excess of 100 mg/L. As such, smaller volumes of water are needed to be pumped to transfer large amounts of oxygen to the culture tank.
The technology also takes up less space when compared to oxygen cones and low-head oxygenators, and if designed properly, one unit can provide oxygen to numerous tanks. As elevated pressure is key to this process, any valve controlling the flow of water and oxygen to a particular tank should be positioned as close to the point of discharge as possible. Placement of control valves upstream from this point can lead to degassing of oxygen in the delivery line and a reduction in the efficiency of the process. The primary disadvantage of this system is the high energy requirement to provide the pressure to dissolve so much oxygen.
(Editor’s Note: This article was originally published in the March/April 2014 print edition of the Global Aquaculture Advocate.)
Author
-

Thomas M. Losordo, Ph.D.
Principal Scientist and Chief Engineer
Aquaculture Systems Engineering
Pentair Aquatic EcoSystems, Inc.
1791 Varsity Drive, Suite 140
Raleigh, North Carolina 27606 USA
Related Posts

Intelligence
Adding value to tilapia to tap into U.S. market
New markets for tilapia and expansion of existing ones can be created by planning and implementing properly designed geographic strategies to meet discriminating consumer preferences. Low labor costs in most producing countries promotes value-adding by the production of fresh fillets.

Innovation & Investment
Brazil study results encouraging for injector
Zero-exchange biofloc systems allow elevated stocking densities and production, but also require more dissolved oxygen and thorough water circulation. A new type of air injector uses only a centrifugal pump to recirculate water while naturally aspirating ambient air.

Responsibility
A look at various intensive shrimp farming systems in Asia
The impact of diseases led some Asian shrimp farming countries to develop biofloc and recirculation aquaculture system (RAS) production technologies. Treating incoming water for culture operations and wastewater treatment are biosecurity measures for disease prevention and control.

Health & Welfare
A comprehensive look at the Proficiency Test for farmed shrimp
The University of Arizona Aquaculture Pathology Laboratory has carried out the Proficiency Test (PT) since 2005, with 300-plus diagnostic laboratories participating while improving their capabilities in the diagnosis of several shrimp pathogens.

