Results show improved fish survival, growth and gill health

The use of lumpfish (Cyclopterus lumpus) is a valuable strategy for biological control in aquaculture due to its appetite for the sea lice (Lepeophtherius salmonis). Efficient control of this major ectoparasite remains one of the most important challenges for the salmon farming industry today.
The number of lumpfish used by the salmon farming industry as sea lice “cleaner fish” has increased exponentially since 2008, and 31 million lumpfish were produced and placed in sea cages in Norway during 2018. The number of cleaner fish hatcheries in Norway, most of them producing lumpfish, has increased from five to 31 in five years.
Although lumpfish appear to be fairly robust between hatching and transfer to sea cages, signs of systemic bacterial infections are frequently observed in hatcheries, and the microbial communities in the rearing water are highly unstable. In addition, the hatcheries have varying survival, ranging from 30 to 90 percent. Currently, lumpfish are produced in flow-through systems (FTS). Knowledge on optimal husbandry and microbial water quality for rearing of lumpfish in land-based production systems is still in its infancy and more research is needed.
This article – adapted and summarized from the original (S.W. Dahle et al. 2020. Production of lumpfish (Cyclopterus lumpus L.) in RAS with distinct water treatments: Effects on fish survival, growth, gill health and microbial communities in rearing water and biofilm. Aquaculture, Volume 522, 30 May 2020) – reports on an investigation of the effects of recirculating aquaculture systems (RAS) and various water treatment design configurations on microbial communities in water and biofilm, microbial environment, survival, growth and gill health of lumpfish. Increased knowledge of the microbial communities created by these systems will be useful for improvement of operational design and sustainable lumpfish production in the future.
This study was funded from RFF Nord (Project number 269204). Partners in the project were SINTEF Ocean, Let Sea AS and Ecomarine Seafarm AS. We would like to thank staff at Let Sea AS and Ecomarine Seafarm AS for practical work during the experiments, Roman Netzer and Deni Ribicic (SINTEF Ocean) for coordination of sequencing.
Study setup
A 146-day experiment with lumpfish was conducted at Ecomarine Seafarm AS at Dønna, Norway, in cooperation with Let Sea AS. Four different treatments were included directly before the water entered rearing tanks, which were all connected to the same RAS loop: 1) RAS without disinfection or filtration for removal of small particles (RAS); 2) RAS with mechanical filtration (20-μm, mechanical filter) (RAS-F); 3) RAS-F with mechanical filtration and a UV unit (RAS-F-UV); and 4) RAS-F-UV with mechanical filtration, UV and an ozone unit (RAS-F-UV-O). In addition, a traditional flow-through system (FTS) was included in the experiment as a reference system. Each treatment included three replicate fish tanks, each 800-liter with conical bottoms and a central bottom drain.
Lumpfish were hatched and fed cryopreserved live feed for the first seven days (Planktonic AS, Norway) and thereafter fed commercial dry feed for lumpfish (Otohime, Japan) and reared in an FTS hatchery the first two months, according to the commercial production procedures at Ecomarine Seafarm. At 0.52 grams, 10,000 lumpfish juveniles were transferred to each tank (6.5 kg per cubic meter) in the on-growing systems used in the experiment. The juveniles were fed continuously with an automatic belt feeder first using one commercial diet (Clean Lumpfish, Skretting AS, Norway) the first two months (pellet size 0.5 to 0.8 mm), then the RAS treatments were fed with a second commercial diet (Lumpfish Grower, Biomar AS, Norway) with increasing pellet size (1.1 to 2.0 mm) for the rest of the experiment.
The fish from the FTS treatment were fed the first commercial diet for 10 days longer than the RAS treatments, due to smaller fish weight, and then the second commercial diet with increasing pellet size. From day 69 the water treatment for RAS was converted to a RAS-F, due to challenges with maintaining the RAS without filtration, because of the need of a heat exchanger, depending on filtration, to lower the temperature. The fish tanks were cleaned once a day by careful siphoning of the walls and bottom of the tanks.
The fish were sorted at days 42 and 71 due to size differences and to maintain an optimal biomass in the tanks (15 to 30 kg per cubic meter). At day 83, the fish (8 to 11 grams) were vaccinated with a commercial vaccine (ALPHA MARINE micro 3.1 vaccine, Pharmaq AS, Norway) with antigens against Aeromonas salmonicida genotype VI, Vibrio anguillarum serotype O1 and Vibrio anguillarum serotype O2a. The experiment ended at day 146 with sampling and monitoring of fish performance, and fish of 59 to 68 grams were transported to sea cages at day 161, with a total production time of 221 days.
For detailed information on the experimental setup; rearing regime; water quality analyses; fish performance; microbial community analyses; and statistical procedures, refer to the original publication.
Results and discussion
To the best of our knowledge, this is the first study to examine the effects of RAS on growth, health, survival and microbial water quality in lumpfish rearing. In addition, it is the first study to compare the effects of different water treatment for individual tanks in the same RAS.
The fish in the RAS treatments showed a significantly higher survival for two of the periods of the experiment, compared to the fish from the FTS (Fig. 1). These results are in accordance with previous studies with marine fish larvae, where RAS resulted in higher survival compared to FTS, and support the hypothesis that lumpfish juveniles reared in RAS will have higher survival compared to siblings reared in FTS. For the two periods with higher survival, the RAS treatments increased survival by an average of 19 percent compared to the FTS.
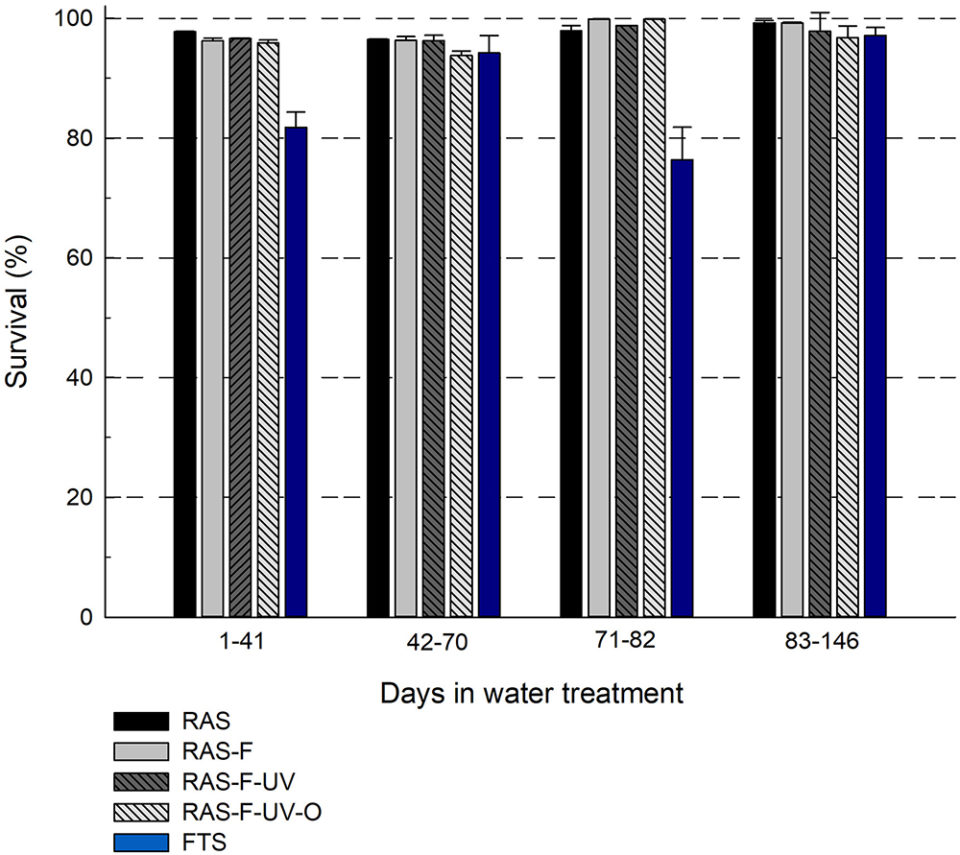
In general, the survival was high for all treatments in the experiment (average 76.0 to 99.9 percent), including the FTS (76.0 to 98.0 percent). Comparably, commercial production of lumpfish in Norway has a lower survival through a production cycle in FTS. The higher survival of fish in FTS in this experiment can be related to the production period. The experiment started two months post-hatch, at which point the typical initial mortality had passed and the fish may be more robust than in the early stages.
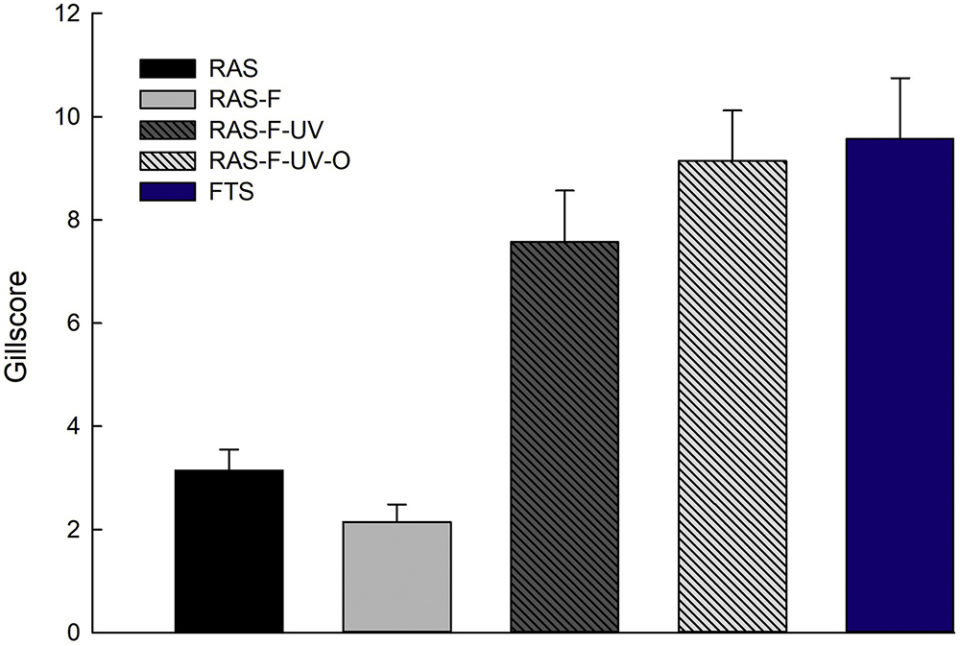
The fish from the RAS treatments without disinfection (RAS and RAS-F) had better gill health than those from FTS and the RAS treatments with disinfection (RAS-F-UV and RAS-F-UV-O). The fish from the RAS-F had the best gill health in this experiment (Fig. 2). This implies that the extra mechanical filtration of the incoming tank water of RAS positively affected the animals. Gill health is an important indicator of fish health and welfare in relation to the farming conditions. The gills take care of important processes like gas exchange, acid-base regulation, excretion of nitrogenous waste, ion- and osmoregulation and hormone metabolism as well as being an important immunological tissue, so their optimal function is of utmost importance for fish health and performance.
The fish grew better in the RAS treatments than in the FTS, due to the significantly higher temperature. RAS is a method for maintaining a stable and optimal temperature year around, whereas FTS depends more on the sea temperature, which will vary through the seasons. During the winter, with seawater temperature dropping below 8 degrees-C, the pathogenic bacterium Moritella viscosa – which causes winter ulcer disease – thrives and is a significant problem. By using RAS facilities and maintaining higher temperatures, it would be possible to prevent the risk of this bacterium.
Disinfection had a significant influence on the bacterial community composition in this experiment, as both UV and ozone change the microbial composition in rearing water and biofilm. Our results indicate that both the UV and the combined UV and ozone treatment changed the microbial community structures. The most abundant bacterial family was the Thiotrichaceae, with the highest abundance in the RAS-F-UV and RAS-F-UV-O treatments (21 to 53 percent).
The RAS and RAS-F treatments had a significantly more diverse and less variable microbial community composition compared to the other treatments at both sampling days, which might indicate a more mature and K-selected (evolutionary strategy of those species that produce few “expensive” offspring and live in stable environments) community in the RAS treatments without disinfection.
As expected, the RAS treatments had significantly higher abundancy of total bacteria in their tank waters than the FTS at both sampling points, probably due to a higher accumulation of particles in the rearing water acting as substrates for the bacteria in the system. The RAS treatments without disinfection had a lower fraction of opportunistic bacteria compared with the RAS treatments with disinfection and the FTS.
Cultured lumpfish live in close contact with the biofilm on the tank walls, as they spend much of the time attached with their ventral suction disc to the tank wall and other surfaces. Biofilm can represent a reservoir for opportunistic bacterial pathogens, so its composition can be important for fish health. Both the RAS treatments and the FTS had a relatively higher abundance of potential pathogens in the water compared to the biofilms. In biofilm, possible pathogenic and problematic bacteria were identified at the highest abundance in the biofilm of the RAS treatments with disinfection, with 19 percent abundance of Moritella from the RAS-F-UV and 33 percent abundance of Leucothrix in the RAS-F-UV-O.
Biofilm microbiota seemed to be less affected by the water treatments, compared to the water microbiota, as the biofilm community varied less between the RAS treatments and especially over time, than the water microbiota. This was expected, since the composition of the layered biofilm is protected against intrusion, like disinfection, and the biofilm is especially protected with surface growth over time. In biofilms, high competition and K-selection may generally be expected, but frequent cleaning or perturbations may open for more r-selecting (evolutionary strategy for species that produce many “cheap” offspring and live in unstable environments) conditions.
Perspectives
In our study, lumpfish were exposed to different microbial communities of both water and biofilm due to different treatments of the incoming tank water. Overall, our results show that lumpfish reared in the RAS treatments were exposed to a more stable microbial community, with a lower share of opportunistic bacteria, which is a probable reason for the higher survival and better gill health of the fish compared to siblings reared in the FTS.
Our results also demonstrate that RAS without disinfection (RAS and RAS-F) had a significantly more diverse and more stable microbial community composition compared to the tanks receiving disinfected RAS water and the FTS. In addition, these treatments had less opportunistic and potential harmful bacteria, which resulted in a better gill health of the fish compared to siblings reared in the RAS with disinfection and FTS. And the fish in the RAS-F had better gill health than fish in the RAS (which was operated without filtration the first 69 days), probably due to the positive effects of reduced particle load.
Altogether, our results indicate that there is a potential to increase lumpfish survival, growth and gill health by producing the fish in RAS, and that RAS with filtration of small particles but no disinfection seem to result in the best fish health and performance. By producing lumpfish using RAS installations, the industry can improve and increase the production to meet the growing demands from the salmon farming industry. The possibility that the earlier stages of lumpfish would benefit even more from being produced in RAS – from hatching and until delivery to sea cages – should be investigated further.
References available from the original publication.
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Authors
-
Stine Wiborg Dahle, M.Sc.
Corresponding author & Ph.D. student
SINTEF Ocean, Department of Environment and New Resources
Brattørkaia 17C, 7465 Trondheim, Norway -
Ingrid Bakke, Ph.D.
Department of Biotechnology and Food Science
Norwegian University of Science and Technology (NTNU)
7491 Trondheim, Norway
-
Mari Birkeland, M.Sc.
Department of Biotechnology and Food Science
Norwegian University of Science and Technology (NTNU)
7491 Trondheim, Norway -
Kristian Nordøy
Let Sea AS
8801 Sandnessjøen, Norway -
Alf S. Dalum, DVM, Ph.D.
Pharmaq Analytic
5008 Bergen, Norway -
Kari J. K. Attramadal, Ph.D.
Department of Biotechnology and Food Science
Norwegian University of Science and Technology (NTNU)
7491 Trondheim, Norway
Tagged With
Related Posts

Aquafeeds
Barnacles to shake up live feeds for aquaculture?
Norwegian startup Planktonic AS believes that it has hit upon a viable alternative to traditional live diets in the form of nauplii from barnacles.

Health & Welfare
Extensive wrasse use keys up ‘cleaner fish’ conservation questions
Few could argue that a reduction in sea lice-fighting chemicals isn't a win for the fish and for the environment. The downside, however, is that increasing numbers of cleaner fish are being caught for use on salmon farms.

Health & Welfare
Animal health giants have sea lice in their crosshairs
Alltech and Benchmark have been working on the next generation of sea lice solutions and believe they have new products that can help salmon farmers win.

Health & Welfare
Healthy fish: Recap of Day 1 at GOAL 2016
Some of the world’s top aquatic animal disease experts shared knowledge about mitigation techniques at the GOAL conference in Guangzhou, China. Topics included sea lice, streptococcus and EHP, which was referred to as the “most insidious” disease impacting shrimp production.


