Ideal pH for most aquaculture species is between 6.0 and 8.5

Pure water ionizes equally into hydrogen ions and hydroxyl ions. If the concentration of hydrogen ion increases, the concentration of hydroxyl ion must decrease and vice versa. Water is acidic if its hydrogen ion concentration is greater than its hydroxyl ion concentration and basic (alkaline) if the opposite is true. Of course, pure water is neutral – neither acidic nor basic.
To avoid the expression of very small concentrations of hydrogen and hydroxyl ions, it is customary to use the pH or negative log of the hydrogen ion concentration as a surrogate for the concentration. The negative logarithm of 10-7 molar, the hydrogen ion concentration of pure water, is 7. This pH is the middle or neutral point of the pH scale.
Waters with lower pH are acidic and those of higher pH are basic. Hydrogen ion concentration increases tenfold for each unit decrease in pH because the scale is logarithmic. For example, hydrogen ion concentrations at pH 6, 5 and 4 are 10, 100 and 1,000 times greater, respectively, than at pH 7. The converse is true for hydroxyl ion concentration.
Natural water pH
The pH of natural waters usually is between 5.0 and 9.0, but lower and higher values sometimes occur. Rainwater normally has a pH around 5.6, because it is saturated with carbon dioxide that has an acidic reaction in water. Lower pH may occur in rainwater because of air pollution – especially contamination of the atmosphere with sulfur compounds from the combustion of fossil fuels that oxidize to form sulfuric acid.
Sulfides in some soils and geological formations oxidize to form sulfuric acid that results in highly acidic conditions (pH 2 to 4) in water that contacts the formations. Highly leached soils are deficient in bases, and water in contact with them will have low alkalinity and pH as low as 5. Waters with high concentrations of humic substances also can have similarly low pH.
Soils may contain basic substances such as limestone, calcium silicate and feldspar that dissolve to increase alkalinity and pH in water. The pH of water tends to increase with greater alkalinity and total dissolved solids. Waters in arid and semi-arid regions typically have pH above 7.5 or 8.0. Normal seawater also has a pH near 8.0.
Acidity, basicity
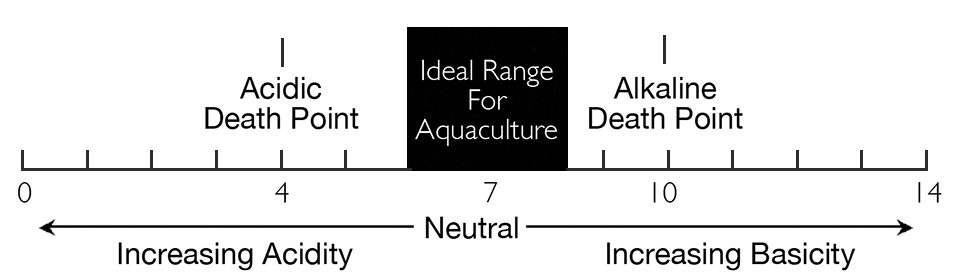
It is important to distinguish between acidity and basicity or alkalinity as defined by the pH scale (Fig. 1) and the water quality variables mineral acidity, total acidity and total alkalinity (Fig. 2). Carbon dioxide normally cannot lower the pH of water below 4.5, and waters with a pH lower than this are said to contain mineral acidity – usually sulfuric acid. Carbon dioxide exists in water up to pH 8.3, so water with pH between 7.0 and 8.3 contains acidity even though it is basic on the pH scale.
The alkalinity of water results from titratable bases in a sample – mainly bicarbonate and carbonate. Bicarbonate can occur in water down to pH 4.5, so water with a pH between 4.5 and 7.0 contains alkalinity despite the fact that it is acidic on the pH scale. Carbonate does not occur in water until the pH rises above 8.3. The alkalinity serves to buffer water against pH change, and a portion of the carbon in bicarbonate is available to plants for use in photosynthesis.
Aquaculture, pH fluctuations
The ideal pH for most aquaculture species is between 6.0 and 8.5 (Fig. 1). Lower pH values may result in slower growth, poorer survival and greater susceptibility to disease in aquaculture species. Brief daily excursions of pH above 8.5 are common in ponds and apparently do not harm aquaculture species. However, long-term exposure to pH of 9.0 or above will have effects similar to those of suboptimal pH. The acid and death points for most species are pH 4 and pH 10, respectively.
Daily fluctuations in pH in ponds result from the net removal of carbon dioxide by plants for use in photosynthesis during the day and the release of carbon dioxide into the water at night by respiration. Because carbon dioxide has an acidic reaction, pH typically is lowest in the early morning. It increases to a maximum during the early afternoon and declines at night (Fig. 3). Large daily pH fluctuations are favored by dense phytoplankton blooms and low-alkalinity, weakly buffered water. Aquaculture ponds typically have dense plankton blooms, so they should be limed if total alkalinity is lower than 50 mg/L.
Daytime pH is often highest in well-illuminated surface water, where photosynthesis is more rapid than in deeper water. The exception is clear water with underwater aquatic weed infestations where pH is greater within the weed beds. Of course, mechanical aeration in aquaculture ponds mixes the water column and often prevents depth-related differences in pH from developing.
When pH rises above 8.3, no free carbon dioxide will be present, but plants can obtain inorganic carbon for photosynthesis from bicarbonate. Removal of carbon from bicarbonate results in the release of carbonate ions into water, and carbonate hydrolysis causes pH to rise further.
In most waters, there is sufficient calcium to limit the carbonate concentration through calcium carbonate precipitation that tempers pH increases. However, in water with low calcium but high total alkalinity concentrations, pH values of 11 or more can occur in the afternoon. Liming materials do not dissolve well in such waters, but calcium sulfate (gypsum) can be applied to increase calcium concentration.
A major reason for a decline in alkalinity in an aquaculture system is nitrification. Ammonia nitrogen – the major nitrogenous waste of aquatic animals – is oxidized to nitrate by denitrifying bacteria. The resulting hydrogen ions neutralize alkalinity, reducing buffering capacity and increasing the possibility of lower morning pH. Routine liming often is needed to maintain adequate alkalinity in highly intensive aquaculture systems, especially those with plastic-lined bottoms and no water exchange.
Alkalinity also can decline in ponds built on acid-sulfate soils bearing pyrite, as the oxidation of pyrite yields sulfuric acid and lowers alkalinity and pH. The remediation of ponds built in acid-sulfate soils is too complex to discuss here.
Measuring pH
The most accurate way to measure pH is with a standard electronic pH meter. Litmus paper only indicates whether water is acidic or basic, and the pH estimated from pH strips only provides an indication of the true pH. Small, pocket-sized pH meters are available from aquaculture supply houses, but these devices are generally unreliable after a short period of use.
It is more reliable to measure pH in situ, because this variable changes quickly during sample storage. The time of day obviously influences the pH, with the lowest values typically found in the morning and the highest between noon and mid-afternoon.
(Editor’s Note: This article was originally published in the July/August 2013 print edition of the Global Aquaculture Advocate.)
Author
-

Claude E. Boyd, Ph.D.
Department of Fisheries and Allied Aquacultures
Auburn University
Auburn, Alabama 36849 USA[117,100,101,46,110,114,117,98,117,97,64,49,101,99,100,121,111,98]
Tagged With
Related Posts

Health & Welfare
A holistic management approach to EMS
Early Mortality Syndrome has devastated farmed shrimp in Asia and Latin America. With better understanding of the pathogen and the development and improvement of novel strategies, shrimp farmers are now able to better manage the disease.

Health & Welfare
A study of Zoea-2 Syndrome in hatcheries in India, part 1
Indian shrimp hatcheries have experienced larval mortality in the zoea-2 stage, with molt deterioration and resulting in heavy mortality. Authors investigated the problem holistically.

Health & Welfare
A study of Zoea-2 Syndrome in hatcheries in India, part 2
Indian shrimp hatcheries have experienced larval mortality in the zoea-2 stage, with molt deterioration and resulting in heavy mortality. Authors considered biotic and abiotic factors. Part 2 describes results of their study.

Health & Welfare
A study of Zoea-2 Syndrome in hatcheries in India, part 3
In this third and final part, authors present recommendations to help reduce the incidence of Zoea-2 Syndrome, which is not caused by any known infectious agents in P. vannamei hatcheries in India.

