Results in a green-water system show interactions with stocking density, natural food availability

The amino acid methionine is very important in various physiological and metabolic processes in animals. Shrimp get methionine and other essential amino acids through their dietary protein or through the breakdown of body protein. Methionine is considered the most limiting essential amino acid in commercial aquafeeds for shrimp. To meet the requirement for methionine, nutritionists formulating shrimp aquafeeds have traditionally depended on intact sources of this amino acid.
But with current efforts to replace fishmeal with other protein sources, an increased use of crystalline amino acids in shrimp aquafeeds is expected, particularly methionine. The dietary requirements for methionine for two commercially farmed shrimp species – Kuruma shrimp (Marsupenaeus japonicus) and black tiger shrimp (Penaeus monodon) – have been estimated at 1.4 percent of the crude protein and at 2.4 percent of the crude protein, respectively. For the Pacific white shrimp (Litopenaeus vannamei), the literature reports a dietary methionine requirement range of 1.9 to 2.9 percent of the crude protein.
In penaeid shrimp, studies of their essential amino acid requirements have typically been carried out in clear-water systems using purified or semi-purified diets, and where the animals had no natural food available. But although the dietary amino acid requirements of shrimp can possibly be affected by several external factors like water quality, feeding regime and stocking density, scant research has examined the possible effect of these external factors with the dietary amino acid requirement in penaeid shrimp.
In this study – which summarizes the original publication in Aquaculture 463 (2016) 16-21 – we evaluated the effect of graded levels of dietary methionine and stocking density on the growth performance of juvenile Pacific white shrimp cultured in a green-water rearing system.

Study setup
The outdoor, experimental rearing system used – 75 polypropylene, 1-m3 round tanks with a bottom surface area of 1.02 m2 – used was previously described by Nunes et al. (2011). Tanks were filled with sand-filtered seawater (30 ± 0.5 ppt salinity), with feed remains and shrimp feces providing the only fertilization needed to achieve green-water conditions. Water was exchanged at 14.2 percent tank volume/day, and continuous aeration provided. Water temperature, salinity, pH and transparency were relatively stable during the experiment: 29.6 ± 0.76 degrees-C, 35 ± 1.4 salinity, 8.05 ± 1.36 and 32 ± 8.7 cm (n = 825), respectively.
L. vannamei postlarvae (PL10) from a commercial hatchery were reared to juveniles (1.97 ± 0.14 g) in nursery tanks, and then stocked at densities of 50, 75 and 100 animals/m2. Shrimp were fed the experimental diets four times daily using 161-cm2 feeding trays (one per tank). The feeding rates were fixed among feeding treatments, but varied during the 10-week long experiment from 4 to 12 percent and based on shrimp body weight. All feed not consumed in the feeding trays was collected, oven-dried, and weighted to calculate the apparent feed intake.
The five experimental diets were designed with minimum inclusions of fishmeal and other marine ingredients, and formulated with varying dietary methionine contents (0.48,0.62, 0.72, 0.81, and 0.94 percent of the diet, on a dry matter basis, DM) and subsequently Met + Cys (methionine + cysteine, 0.96, 1.09, 1.19, 1.28 or 1.40 percent DM). Each treatment had five replicate tanks, for a total of 75 experimental tanks in the outdoor rearing system.

At the start, 100 shrimp were randomly collected, and 50 shrimp from each dietary treatment were also collected at the end of the study. Caudal muscle and hepatopancreas from the sacrificed animals were collected and processed for analysis. Natural food (including aquafeed remains, algal material and detritus) samples were also collected from tank walls from each dietary treatment, and similarly processed. All samples were analyzed for dry matter and crude protein following standard methods, and the amino acid concentrations in the experimental diets, natural food, and shrimp tissues and organs were analyzed.
All shrimp were individually weighed at stocking and at harvest to determine their initial and final body weight (g), weekly growth rate (g/week), specific growth rate (SGR, g/week), yield (g of shrimp gained/m2), and final survival (%).
For additional details on the experimental diets and data analysis procedures, please refer to the original publication.
Results
In our study, the growth performance of L. vannamei juveniles was significantly affected by shrimp stocking density and dietary methionine (Met) content, possibly as a result of natural food availability. The higher the stocking density, the more dietary Met was required to improve shrimp growth performance. Final survival of shrimp was over 90 percent irrespective of dietary Met and stocking density, but a significant reduction in survival resulted when 75 shrimp/m2 were fed the 0.48 percent Met diet (0.96 percent Met + Cys; P b 0.05).
Final shrimp body weight declined with higher stocking densities, and FCR worsened as stocking density increased. Table 1 summarizes our study data on growth performance and feed utilization of the juvenile L. vannamei fed with different dietary methionine (Met) levels and under different stocking densities.
Facanha/Nunes, Table 1
| Performance (1) | % Met | Shrimp stocking density: 50 shrimp m-2 | Shrimp stocking density: 75 shrimp m-2 | Shrimp stocking density: 100 shrimp m-2 |
|---|---|---|---|---|
| Final survival (%) | 0.48 | 94.1 ± 5.7 | 83.1 ± < 0.001a | 91.8 ± 5.5 |
| Final survival (%) | 0.62 | 98.8 ± 1.1 | 96.9 ± 3.3ab | 92.5 ± 8.0 |
| Final survival (%) | 0.72 | 93.3 ± 7.5 | 97.7 ± 1.4b | 93.5 ± 8.1 |
| Final survival (%) | 0.81 | 98.5 ± 1.0 | 90.3 ± 6.8ab | 91.2 ± 4.2 |
| Final survival (%) | 0.94 | 95.6 ± 1.9 | 95.1 ± 2.0b | 95.4 ± 3.2 |
| Growth rate (g week-1) | 0.48 | 1.36 ± 0.04aA | 1.39 ± 0.04abB* | 1.29 ± 0.06C*†§ |
| Growth rate (g week-1) | 0.62 | 1.56 ± 0.04bA* | 1.32 ± 0.06aB†§ | 1.33 ± 0.08B† |
| Growth rate (g week-1) | 0.72 | 1.49 ± 0.11bcAB† | 1.41 ± 0.05abA* | 1.36 ± 0.06B*† |
| Growth rate (g week-1) | 0.81 | 1.41 ± 0.04ac | 1.46 ± 0.04ab | 1.36 ± 0.03* |
| Growth rate (g week-1) | 0.94 | 1.46 ± 0.06ab§ | 1.41 ± 0.05ab* | 1.37 ± 0.04* |
| AFI (g stocked shrimp-1) | 0.48 | 21.0 ± 0.4a | 20.7 ± 0.1a | 21.1 ± 0.2 |
| AFI (g stocked shrimp-1) | 0.62 | 21.4 ± 0.1ab | 21.2 ± 0.3ab | 21.2 ± 0.1 |
| AFI (g stocked shrimp-1) | 0.72 | 21.4 ± 0.1a | 21.3 ± 0.1ab | 21.3 ± 0.2 |
| AFI (g stocked shrimp-1) | 0.81 | 21.5 ± 0.1b | 21.4 ± 0.3b | 21.4 ± 0.2 |
| AFI (g stocked shrimp-1) | 0.94 | 21.2 ± 0.1ab | 21.1 ± 0.3ab | 21.3 ± 0.1 |
| FCR | 0.48 | 1.66 ± 0.08aA | 1.84 ± 0.06aB | 1.81 ± 0.08B |
1AFI, apparent feed intake; FCR, food conversion ratio
The dietary Met inclusion levels needed to attain maximum shrimp growth under green water conditions ranged between 0.72 and 0.82 percent of the diet (on a dry matter basis, or 1.19 and 1.28 percent Met + Cys), i.e.,1.98 and 2.29 percent of the dietary crude protein (CP) for densities under 50, 75 and 100 animals/m2, respectively. These values are similar to those reported for other commercially-farmed penaeid shrimp.
A large portion of the published literature on the essential amino acid (EAA) requirements of penaeid shrimp comes from studies in controlled clear-water conditions and without natural food. Our results show that the natural food from tank walls had as much as 26 percent CP and 0.31 percent Met, and that increased dietary Met levels were required with higher shrimp stocking densities.
The availability and nutritional composition of natural food may affect the need for higher dietary Met inclusion levels in shrimp aquafeeds. Higher shrimp biomasses exert more demand on natural food in green-water systems, resulting in a higher dependence on aquafeed nutrients to support shrimp growth and survival.
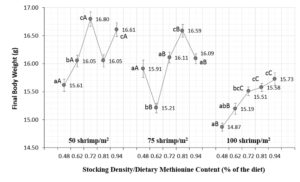
Methionine is one of the most important amino acids in aquatic feeds, serving as a sulfur source in some reactions and affecting the activity of key enzymes. In our study, dietary Met beyond 0.81 percent (1.28 percent Met + Cys) did not improve the final body weight of shrimp, and we did not detect any adverse effects (Figure 1).
As previous studies with L. vannamei that have not reported any relation between dietary amino acid levels and tissue composition, shrimp feed intake and final shrimp survival in our study were not negatively affected under higher dietary Met levels, and increased dietary Met did not result in higher deposition in shrimp muscle.
Perspectives
Results from our green water study show an interaction between dietary Met content and the stocking density of shrimp, driven by natural food availability, but the Met requirements for Pacific white shrimp remain to be clearly defined.
The Met inclusion in practical aquafeeds for shrimp cultured in systems with natural food sources should vary between 0.72 (1.19 percent Met + Cys) and 0.81 percent Met (1.28 percent Met + Cys) for animal densities of 50-75 shrimp/m2. The specific availability of natural food and its composition may indicate the need for higher or lower inclusion levels in aquafeed.
References available from the senior author.
Authors
-
Felipe N. Façanha, M.Sc.
LABOMAR – Instituto de Ciências do Mar
Universidade Federal do Ceará
Avenida da Abolição, 3207 – Meireles
Fortaleza, Ceará, 60.165-081, Brazil -
Dr. Adhemar R. Oliveira-Neto
Evonik Degussa Ltda.
Alameda Campinas, 579 - 10° andar São Paulo
São Paulo, 01.404-000, Brazil
-
Dr. Claudia Figueiredo-Silva
Evonik Nutrition & Care GmbH
NC, 10-B531, Postfach 1345
Rodenbacher Chausse 4
63404 Hanau, Germany -
Alberto J.P. Nunes, Ph.D. (corresponding author)
LABOMAR – Instituto de Ciências do Mar
Universidade Federal do Ceará
Avenida da Abolição, 3207
Meireles, Fortaleza, Ceará, 60.165-081, Brazil
Tagged With
Related Posts

Aquafeeds
Replacing fishmeal with DFB in pompano diets
A study evaluated the inclusion of bacterial, dried fermented biomass as a replacement for fishmeal in four practical diets for Florida pompano juveniles. There were no significant differences in final weight, survival, FCR or thermal-unit growth coefficient.

Aquafeeds
The digestibility of hydrolyzed soy protein
Studies demonstrate that hydrolyzed soy protein can be well digested and utilized by Pacific white shrimp, and that digestibility is improved through a bioprocess with the bacterium Lactobacillus spp.

Health & Welfare
Dietary acidification in aquaculture
Much of the chemical breakdown of foodstuffs begins in the stomachs of animals through acidification. Effective use of digestive biology is a goal in aquaculture, so dietary acidifiers have been gaining interest in recent years.

Health & Welfare
Dietary organic acids used as growth promoters, anti-microbials
A feeding trial measured growth, nutrient utilization and faecal/gut bacterial counts in triplicate groups of red hybrid tilapia (Oreochromis sp.). Study results show that dietary organic acids can potentially replace OTC as a growth promoter and anti-microbial in tilapia feeds.


