Fast, simple test based on the sensitivity of nitrifying bacteria

Synthetic polymers such as plastic and synthetic rubber are widely used in aquaculture as tank and pond liners, and solid support media for nitrifying biofilters. Synthetic polymer products, however, pose potential toxicity that can endanger the health of cultured animals and interfere with the biological nitrification process.
Not all synthetic polymers leach toxic chemicals, and most plastics and rubbers are generally inert to most biological forms. Where identified, the toxicity is usually the result of a slow release of molecules embedded in the synthetic polymers during their manufacture.
Due to the proprietary nature of the manufacturing process, it is difficult to obtain detailed technical information about the various chemical additives used during the production of polymers. Therefore, toxicity screening is recommended for synthetic polymer products prior to their application in aquaculture.
Potential toxicity
The potential toxicity of synthetic polymers to living organisms is affected by the types and amounts of chemical additives used during their production, as well as the rates at which these molecules leach out of the polymers into the surrounding water. The rates of release are affected by the sizes of the migrating molecules, and water temperature, pH and salinity.
Toxicity testing
To predict and consequently avoid problems resulting from the potential toxicity of synthetic polymer products used in aquaculture operations, the authors developed a fast, simple toxicity test based on the sensitivity of nitrifying bacteria to the products. The test can determine the deleterious effects that synthetic polymers have on the nitrification process, and alert the manufacturer and culturist of potential risks to aquatic animals. Incorporating one or two polymer samples, 10-day incubation, two controls and six replicates, the test costs about $380.
Sensitive bacteria

Generally, bacteria are less sensitive to toxic chemicals than multicellular organisms and thus are not used in standard toxicity tests. However, nitrifying bacteria were shown to be even more sensitive to polymer toxicity than shrimp in certain cases, which presents the opportunity to use an inexpensive and rapid microbial test instead of animal tests.
Nitrifying microorganisms perform the process of nitrification, the biological oxidation of ammonia and nitrite, which is crucial in keeping the toxic nitrogenous compounds in aquaculture at low levels. The nitrifying bacteria used in the toxicity test are autotrophs from a small number of species that include the ammonia-oxidizing and nitrite-oxidizing bacteria groups.
They are strictly aerobic, consume carbon dioxide as their sole carbon source, and obtain energy via the oxidation of nitrogen. During the ammonia oxidation process, the bacteria produce acid that must be neutralized. In general, nitrifying bacteria are slow growers, are highly sensitive to various chemicals, and have a narrow pH range in which their activity takes place.
The toxicity test is performed by exposing nitrifying microorganisms to samples of synthetic polymers and tracking the microorganisms’ ability to oxidize ammonia and nitrite. Toxicity is measured with the time of exposure of the microbial cultures to the polymer samples and the level of impairment of the nitrification process in the presence of a tested product.
Examples of use
The test has been effectively used in a variety of applications that evaluated synthetic polymer products.
Shrimp mortality from tank liners
Episodes of shrimp mortality occurred during the growth of white shrimp (Litopenaeus setiferus) in a superintensive recirculating production system. The ethylene propylene diene monomer (EPDM) synthetic rubber liner used to line the shrimp tanks was suspected of being toxic to the shrimp.
The EPDM liner material was tested under controlled conditions with shrimp grown in small tanks in the presence or absence of the liner. In the presence of the liner, all the shrimp died within two weeks. Control shrimp not exposed to the liner had 100 percent survival during the same period. Additionally, ammonia accumulation was noted in the tanks that contained the liner material.
The microbial toxicity test of the liner used nitrifying bacteria in the absence of shrimp. Standard cultures of a mixed nitrifying bacterial population were maintained in a specific nitrification salts medium, aerated, and exposed to the liner for 50 hours.
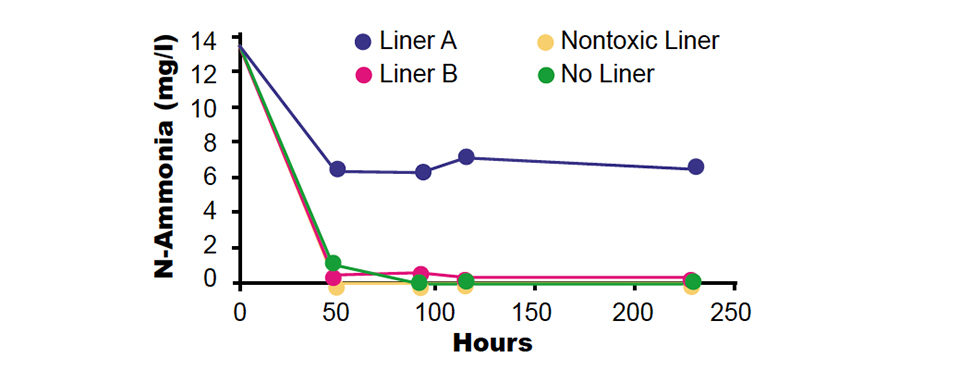
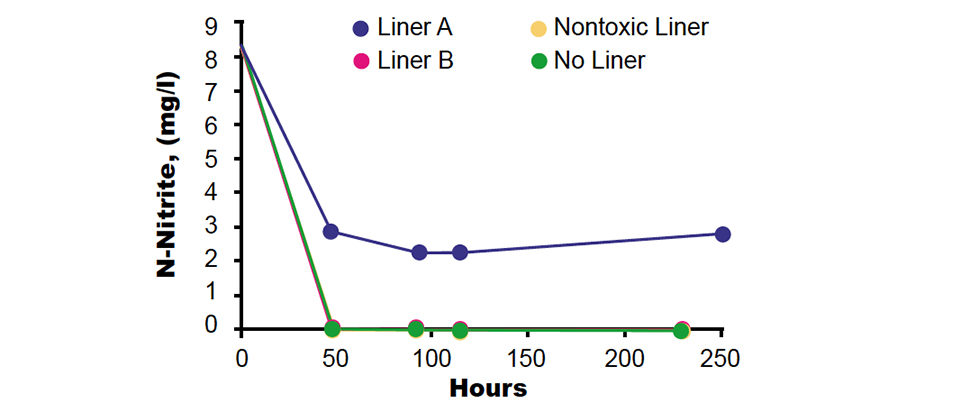
In the presence of the liner, nitrification stopped within a few hours and left behind a constant presence of ammonia and nitrite, whereas the no-liner controls demonstrated fast and efficient ammonia and nitrite removal. The EPDM liner apparently released chemicals that were toxic to both shrimp and nitrifying bacteria. Chemical additives released from EPDM can include monomers, benzoyl peroxide, aldehyde amines, thiozoles, aromatic diamines, aniline, and different oils.
Bead selection for biofilters
Polyurethane beads were considered as solid support media for nitrifying microorganisms in a biofilter for a recirculating system raising tilapia. The beads seemed suitable for aquaculture, and had no deleterious effect under normal microbial growth conditions. However, relatively minor changes in temperature (23 to 27 degrees-C) and/or pH (7.34 to 7.80) caused the polymer to strongly inhibit nitrification.
Although the temperature and pH were well within the operating zones of the nitrifying culture, the results implied an increased release of toxic compounds from the beads due to the pH and temperature changes. The toxic polyurethane biofilter was replaced with a nontoxic polymer before damage to animals took place.
Chemical additives that may be released from polyurethane include tetrahydrofurane and adipic acid. Even though some types of polyurethane produced with little or no additives are considered not toxic and used for medical purposes, one can not assume that all polyurethane products are nontoxic. When purchasing new beads for biofilters, it makes sense to test them by a microbial toxicity test prior to use.
Safer EPDM liner products
EPDM sheets are an attractive source for liners in aquaculture. Although this material is a little more expensive than high-density polyethylene (HDPE), the ease of installation and repair of EPDM offsets the higher initial cost.
To help choose a safe EPDM membrane formulation for pond/tank liners in shrimp aquaculture, the microbial toxicity test was applied to evaluate the potential toxicity of synthetic rubber membranes made of EPDM polymers with different fillers, additives, and curing agents. A nontoxic HDPE liner previously used for several months in a shrimp-growing tank was the control for no toxicity. A control with no liner was also used.
Some of the EPDM membrane samples exhibited no negative effect on nitrification at all exposure times. Other samples were toxic to ammonia oxidation, and some were also found to inhibit nitrite oxidation. Figs. 1 and 2 present the results of the short-term screening tests of two representative EPDM membrane samples. Sample A was toxic and sample B was nontoxic to nitrifying bacteria.
(Editor’s Note: This article was originally published in the April 2005 print edition of the Global Aquaculture Advocate.)
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Authors
-
Ami Horowitz, Ph.D.
UPAH Tech, Inc.
3666 Stoer Road
Shaker Heights, Ohio 44122 USA -
Sarah Horowitz, Ph.D.
UPAH Tech, Inc.
3666 Stoer Road
Shaker Heights, Ohio 44122 USA -
Tzachi M. Samocha, Ph.D.
Mariculture Research Facility
Texas Agricultural Experiment Station
Texas A & M University System
Corpus Christi, Texas, USA
Tagged With
Related Posts

Health & Welfare
Biosecurity for shrimp farms
With the global spread of viruses, biosecurity has become an essential element of every shrimp farm. Biosecurity starts with quality of farm design.

Health & Welfare
Brunei project develops technology for large black tiger shrimp production, part 1
A five-year project was undertaken in Brunei Darussalam to develop advanced technology for the production of large black tiger shrimp. A combination of technologies has enabled efficient production of large-sized black tiger shrimp, which could lead to a resurgence of this species in Asia.

Health & Welfare
Digestibility enhancer can offset cholesterol content in shrimp feed
Cholesterol is an essential nutrient for penaeid shrimp. Trials found that a digestibility enhancer based on natural emulsifiers was as effective as purified cholesterol in improving shrimp growth and feed conversion.

Responsibility
For the future, a feed that makes fish feces float?
German researcher believes that floating fish feces – stemming from cork used in feed blends – would help recirculating aquaculture systems more efficiently remove suspended solids. The big question is, will feed manufacturers get on board?


