Two decades of study on shrimp, insect viral defense mechanisms open the way for vaccine production and heritable anti-viral immunity

This article contains breaking news of a discovery that should help us design new shrimp vaccines and to tailor specific pathogen free (SPF) shrimp stocks to tolerate all of the major shrimp viruses. From the outset, I need to make it very clear that the viruses I refer to are problematic only for shrimp and for shrimp farmers whose shrimp production may be negatively affected by them. These viruses do not infect vertebrates, including humans, and so pose no threats to human or animal health, except for shrimp and perhaps other crustaceans.
In 1998, I and my student Tirasak Pasharawipas (now a Professor at the Medical Science Department of Rangsit University in Thailand) formulated a proposal that shrimp and insects defended themselves against viruses by tolerating them instead of expunging them. We called the phenomenon “viral accommodation.” Although few people agreed with us at the time, our research group at Centex Shrimp persisted with our research and proved that shrimp and insects could accommodate one or more viruses for up to a lifetime without negative effects. However, we didn’t know the underlying mechanism that made this possible. All we knew was that shrimp didn’t have antibodies and that something else must be at work.
Then in 2009, I had my “eureka moment” based on scattered evidence and hypothesized a new mechanism by which shrimp and insects could use DNA and RNA instead of antibodies to defend against viral pathogens (Flegel, T.W., 2009. Hypothesis for heritable, anti-viral immunity in crustaceans and insects. Biology Direct. 4, 32; doi: 10.1186/1745-6150-4-36). Fig.1 illustrates the 2009 hypothesis for the mechanisms underlying the phenomenon of viral accommodation. I suggested several ways to test this hypothesis with predicted outcomes that would either support or counter the hypothesis.
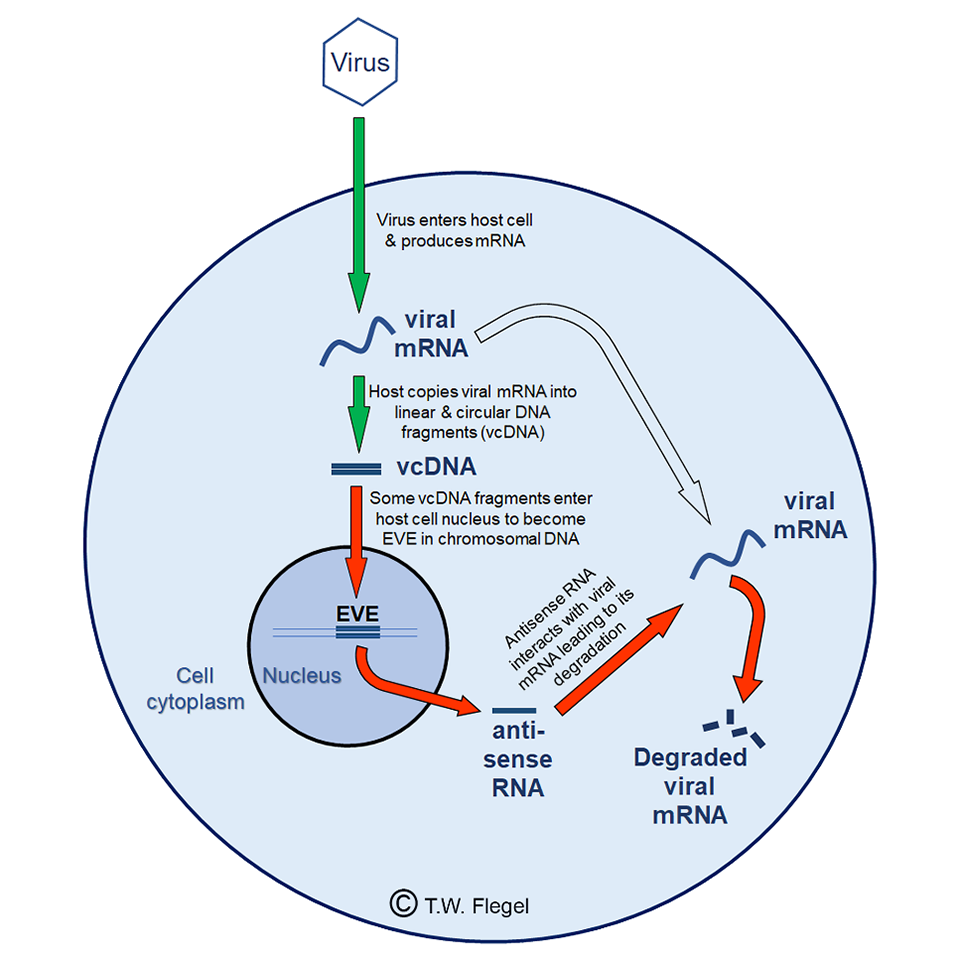
Scientists in France (the group of Dr. Maria Carla Saleh at the Pasteur Institute, Paris, France) and in the United States (the group of Dr. Raul Andino at the University of California, San Francisco, Calif., USA) working with insects (fruit flies and mosquitoes) from around 2013 to 2020, proved that the key elements of the 2009 hypothesis were correct for several RNA viruses. Basically, to accommodate viruses, shrimp and insects copy fragments of viral RNA into DNA and insert the DNA copies into their own genome. These copy fragments are called EVE (endogenous viral elements) that make anti-sense RNA [a single-stranded RNA that is complementary to a protein coding messenger RNA (mRNA) with which it hybridizes, and thereby blocks its translation into protein]. The anti-sense RNA leads to degradation of viral mRNA, thus keeping the virus in check. If the EVE occurs in eggs or sperm, they are passed on to the next generation. This is a big advantage over antibodies that cannot be passed on to offspring.
One surprising result from the research on insects in France was that the DNA fragments copied from viral RNA occurred in both linear and circular forms. This was not predicted in my hypothesis of 2009 in which I envisioned only linear DNA copies. The French report described how the circular viral copy DNA (cvcDNA) could be specifically extracted from insects, and how injection of the cvcDNA extract could protect naïve mosquitoes from death upon viral challenge. I was very excited about these results because circular DNA is very stable, and it suggested that cvcDNA had the potential to serve as a shrimp viral vaccine that would be able to survive feed processing for oral delivery. Although all the insect work was done with RNA viruses, we hypothesized, based on my 2009 hypothesis, that the mRNA of DNA viruses could also serve as the target for production of cvcDNA.
It should be possible to develop shrimp stocks tolerant to all major viruses.
In the past few months, our group in Thailand has been able to follow the insect protocols to extract cvcDNA from shrimp infected with a DNA virus and to show that it can protect naïve shrimp against that virus. Specifically, we used black tiger shrimp (Penaeus monodon) infected with IHHNV (Infectious Hypodermal and Hematopoietic Virus, recently renamed Penstylhamaparvovirus 1). We were able to extract IHHNV-cvcDNA from the infected shrimp and inject it into Pacific whiteleg shrimp (Penaeus vannamei), where it prevented IHHNV replication. A preprint of this work is already available online at the BioRxiv website for those who are interested in the details. The results of this work open the way for the possible development of cvcDNA vaccines for shrimp.
When the cvcDNA extract we obtained from IHHNV-infected P. monodon was sent for next generation sequencing, NGS [a DNA sequencing technology that revolutionized genomic research, significantly reducing the time needed to sequence genomes], we were able to prove that the extract contained a variety of cvcDNA types that originated from IHHNV. This provided final confirmation that IHHNV-infected shrimp could produce cvcDNA, similarly to the insects infected with RNA viruses.
However, we also made the very surprising discovery that some of the cvcDNA sequences in our extract came from an EVE that had previously been described from P. monodon in Madagascar as “non-infectious IHHNV” by Professor Don Lightner’s group in 2006. These results are included in the preprint at the BioRxiv website mentioned above. Some of these unexpected cvcDNA types included IHHNV and host gene sequences linked together, confirming their origin from the host shrimp genome. We found this even more exciting, because it indicated that cvcDNA extracts from shrimp could be used to screen shrimp for the presence of EVE.
https://www.aquaculturealliance.org/advocate/donald-lightner-influential-figure-in-shrimp-aquaculture-remembered/
As stated above, the American and French scientists working on insects have proven that some EVE produce antisense RNA that can protect against diseases caused by the homologous virus. I call these protective-EVE. We had already proven in 2011 and 2017 that EVE with high-sequence identity to extant IHHNV and WSSV occurred in shrimp and could be inherited. However, we did not have any way to easily determine which shrimp carried EVE for any particular virus or whether the EVE were protective. Now, it appears we have an easy way to do this by simply sequencing cvcDNA extracts from apparently uninfected shrimp.
We have already begun to screen individual broodstock specimens from stocks of SPF P. monodon and P. vannamei for the presence of EVE from shrimp viruses by sequencing their cvcDNA. For example, we are screening for WSSV-EVE in SPF stocks that include WSSV negative founder shrimp that arose from ancestors with probable exposure to extant types of WSSV.
Anyone can do this type of experiment. The discoveries that cvcDNA arises upon shrimp viral infection or originates from EVE are simply revelations of natural phenomena. Thus, they are un-patentable and anyone is free to seek applications based on these discoveries. Shrimp broodstock producers may take immediate advantage of cvcDNA extraction and sequencing to screen their stocks for potentially protective EVE. Once identified, they can subsequently follow and preserve those EVE in their breeding programs simply by using polymerase chain reaction [PCR, a widely used technique to rapidly make millions to billions of copies of a specific DNA sample, where scientists can use a very small sample of DNA and amplify it (or a part of it) to a large enough amount to study in detail] methods. In this way, we believe it should be possible to develop shrimp stocks tolerant to all major viruses.
In addition, it is possible that protective cvcDNA arising from EVE or from viral infections may be developed into shrimp vaccines produced by PCR for use in hatcheries and grow-out ponds. They could also be used for injection into the ovaries of domesticated breeding stocks to give rise to heritable, protective EVE in their offspring. This technology should also apply to domesticated insects such as silkworms and honeybees. It is true that the offspring would constitute transgenic organisms, but the processes by which they arise are natural and already occur in nature. Basically, we would simply be selecting the most effective of the already-existing EVE and ensuring that they are present in our breeding stocks.
Essentially shrimp, insects and perhaps other invertebrates have been interacting with viruses in this way using ancient natural mechanisms for autonomous genetic modification (AGMO). Technically speaking, this means that they are natural transgenic organisms (NTO) with which humanity has been interacting for millennia without negative effects. Their cvcDNA constructs consist of naked DNA that is not replicative, so they cannot independently increase in number and spread like the viruses from which they originate.
This AGMO animal system for interacting with viruses is predated by a somewhat similar AGMO system (i.e., the well-known CRISPR mechanism – a technology that can be used to edit genes) that was probably used by archaebacteria [the oldest living organisms on earth] and eubacteria [the “true” bacteria, single-celled prokaryotic (typically unicellular organism without a nuclear membrane-enclosed nucleus) microorganisms – includes all types of bacteria except for archaebacteria] to control their viral pathogens (bacteriophages; viruses that infect and replicate within all bacteria) even before the existence of protozoans. Thus, AGMO and NTO are much more common than previously realized, and there is no need to fear this technology.
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Author
-

Timothy W. Flegel, Ph.D.
Center of Excellence for Shrimp Molecular Biology and Biotechnology (Centex Shrimp)
Faculty of Science
Mahidol University
Rama VI Road, Bangkok 10400, Thailand; and
National Center for Genetic Engineering and Biotechnology (BIOTEC)
National Science and Technology Development Agency (NSTDA)
Thailand Science Park
Pathum Thani, 12120 Thailand

