TSV attacks at low salinity are more critical due to scarcity of minerals for molting

Since Taura syndrome virus (TSV) was first recognized as a shrimp disease in 1992, it has caused serious economic losses to shrimp farmers throughout the world. Efforts in Thailand and the United States have been successful in controlling this disease through selective breeding programs to improve resistance. However, few protocols for controlling TSV infection in ponds have been reported.
Although Taura syndrome can appear in all culture phases of shrimp, the highest mortality usually occurs during the first month of the growout phase. Infected juveniles show pinkish to reddish color followed by black cuticular lesions, empty guts and expanded chromatophores. Histopathological exams of infected shrimp have shown multifocal to extensive areas of necrosis in the sub-cuticular epithelium, connective tissue and adjacent striated muscle.
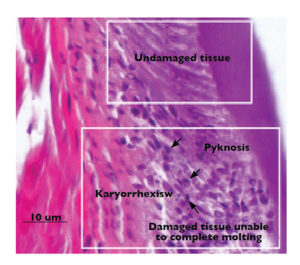
Affected cells often display nuclear pyknosis and karyorrhexis (Fig. 1). Due to this damage in the cuticular and sub-cuticular tissues, shrimp lose the ability to molt or may die in the process. Only after these tissues have recovered and are capable of using the minerals needed for molting can shrimp overcome the disease.
TSV treatment
The authors have established a procedure to reduce the impacts of Taura syndrome virus (TSV) in the culture of Pacific white shrimp (Litopenaeus vannamei). This procedure focuses on avoiding the molting process of shrimp by limiting culture conditions.
Once mortality is detected and TSV has been identified through histopathology, real-time polymerase chain reaction or in situ hybridization, actions must be taken to keep shrimp from molting. These include zero water exchange, reduction of feed, increasing pH to 8.0 or above and strong aeration to keep optimum water quality.
Feeding infected shrimp normally can cause molting and mortality thereafter. Therefore, in many cases, feeding should be reduced or stopped. The use of feed trays can help evaluate consumption and mortality, since moribund shrimp often gather in the feed trays before mortality occurs.
To keep pH at or above 8.0, liming treatments with calcium carbonate or calcium hydroxide should be applied. Every day, pH readings should be taken at dawn, when the lowest pH level occurs and decisions on applying lime can be made. Application of other chemicals like fertilizers or antibiotics should be avoided.
It is expected that a TSV attack at low salinity will be more critical due to the scarcity of minerals for molting. This has been noticed in field visits by the authors to low-salinity farms affected by TSV in Thailand and China.
Moribund and dead shrimp should be collected from the pond every day. This will avoid further deterioration of the water quality and disease propagation through cannibalism. The most efficient way to collect dead shrimp is through the discharge gate or siphoning.
Once the critical period of four to five days has passed and mortality stops, feed dose and water exchange should be resumed gradually. Remains of TSV-affected areas can be readily seen in the shells of animals that have molted after a TSV attack. Usually, full recovery of shrimp can take two consecutive molts.
Treatment trial
The authors’ procedure was tested in intensive culture on Naozhou Island in China’s Guangdong Province during the summer cycle of 2009. A marked difference in productivity was noted between the control and TSV treatment ponds (Table 1).
Ching, Average results of ponds with treatment against Taura syndrome infection, Table 1
| Area (ha) | Stocking Density (shrimp/m²) | Yield (kg/ha) | Weight (g) | Survival (% | Feed-Conversion Ratio | Time (days) | |
|---|---|---|---|---|---|---|---|
| Control* | 0.64 | 146.8 | 4,770 | 13.0 | 25.4 | 2.54 | 87 |
| Treatment* | 0.75 | 147.0 | 11,540 | 15.7 | 50.3 | 1.26 | 78 |
Table 1. Average results of ponds with treatment against Taura syndrome infection (Treatment) and ponds without treatment (Control) in intensive shrimp culture in China.
The trial area was affected by heavy typhoon rain at the end of the culture cycle. In spite of using up to 200 kg/ha of lime to increase pH, this could not be achieved, and mortality occurred prior to harvest. Due to the rain, survival in the treated ponds dropped from 80 to 50 percent.
(Editor’s Note: This article was originally published in the May/June 2011 print edition of the Global Aquaculture Advocate.)
Authors
-
Carlos A. Ching
Technical Manager
Nicovita – Alicorp SAA
Av. Argentina 4793
Callao, Peru[101,112,46,109,111,99,46,112,114,111,99,105,108,97,64,109,103,110,105,104,99,99]
-
Chalor Limsuwan, Ph.D.
Professor
Department of Fishery Biology
Kasetsart University
Bangkok, Thailand
Related Posts

Health & Welfare
New WSSV, TSV genotypes identified in Saudi Arabia
To determine origins of White Spot Syndrome Virus and Taura Syndrome Virus in Saudi Arabia, authors performed genotyping studies and found a new genotype in each of the isolates collected.

Health & Welfare
Study: TSV exposure may lessen YHV effects in white shrimp
Since Taura syndrome virus was in the Americas when yellow head virus become pandemic in Asia, it is possible TSV prevented shrimp in the Western Hemisphere from infection by materials from Asia containing YHV. To test this idea, the authors evaluated the viral interactions in shrimp preinfected with TSV and then challenged with YHV.

Health & Welfare
Ammonia addition enhances microbial flocs in nursery phase for Pacific white shrimp
In a study, “pre-fertilization” in the nursery phase of a biofloc system for shrimp was tested. The objective was to accelerate the biofloc formation to minimize ammonia concentrations, avoiding high peaks during culture.

Aquafeeds
A look at protease enzymes in crustacean nutrition
Food digestion involves digestive enzymes to break down polymeric macromolecules and facilitate nutrient absorption. Enzyme supplementation in aquafeeds is a major alternative to improve feed quality and nutrient digestibility, gut health, compensate digestive enzymes when needed, and may also improve immune responses.


