Nitrogen fertilizers can stimulate phytoplankton growth, increase natural food base

Nitrogen is essential to all living organisms mainly because it is a component of amino acids that make up protein. It is second only to phosphorus in its importance as a nutrient-limiting factor for plant growth. Plants use ammonium and nitrate as nitrogen sources. Protein from plants is the source of organic nitrogen and essential amino acids for animals. Bacteria decompose the remains of dead plants and animals, and rely on these residues for organic nitrogen and essential amino acids.
Molecular nitrogen comprises about 78 percent of the volume of gases in the atmosphere. Surface waters typically contain 10 to 15 mg per liter dissolved molecular nitrogen. Nitrogen gas is relatively inert and unavailable as a nutrient to most organisms. Some blue-green algae and bacteria, however, can reduce molecular nitrogen to ammonia for use in the synthesis of amino acids and protein, a process known as nitrogen fixation. Nitrogen-fixing microorganisms become a source of plant-available nitrogen when they die and decay.
Roles in aquatic production
Fig. 1 depicts the nitrogen cycle. Nearly all aspects of this cycle operate in aquaculture ponds and profoundly influence water quality. Nitrogen fertilizers such as urea or ammonium nitrate can be applied to ponds to stimulate phytoplankton growth and increase the natural food base for fish or shrimp production.
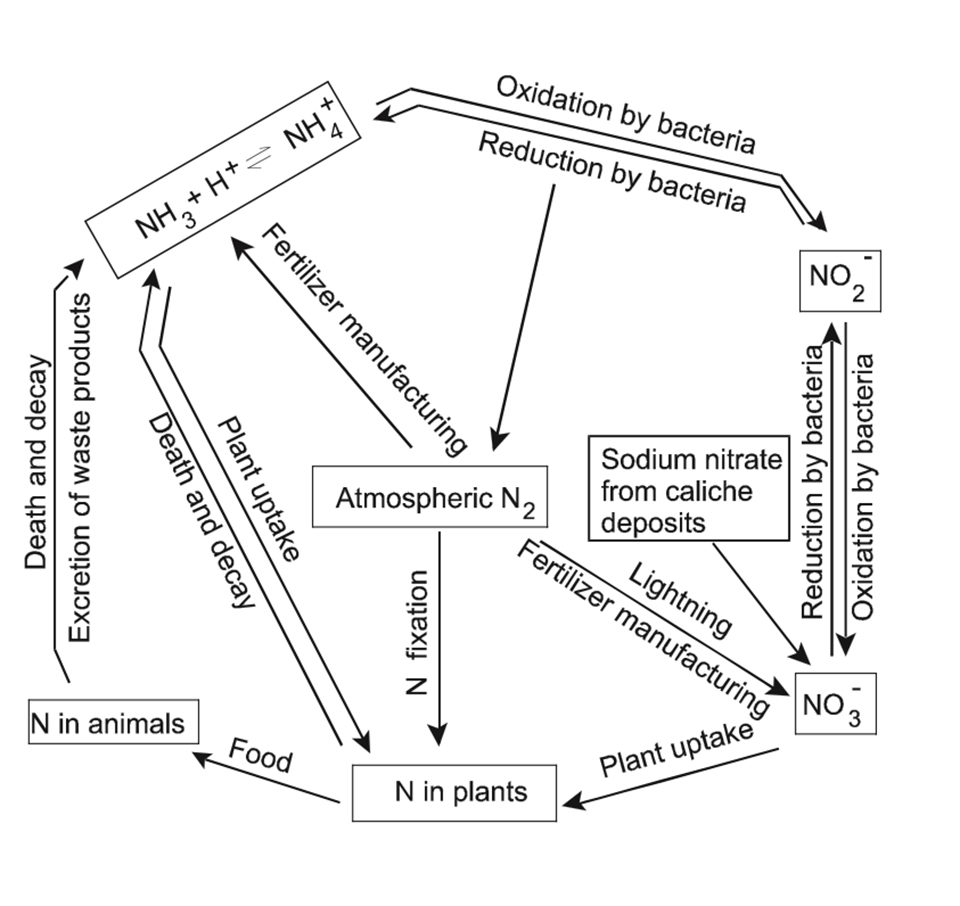
Urea is an organic compound, but it hydrolyzes in water, yielding ammonia and carbon dioxide. Of course, phosphorus fertilizer usually is applied simultaneously with nitrogen fertilizer. When organic fertilizers are applied to ponds, they are decomposed by bacteria, and ammonia and phosphate are released.
Aquatic animal production can be increased greatly above that possible in fertilized ponds by using high-quality feeds that contain 25 to 40 percent crude protein. Cultured animals convert 20 to 40 percent of feed nitrogen into biomass. Much of the rest is converted to ammonia by microorganisms that decompose uneaten feed and feces and by excretion of ammonia by the culture species.
Ammonia exists in water in a pH- and temperature-dependent equilibrium with ammonium. Analytical methods for ammonia measure both ammonia and ammonium or total ammonia nitrogen (TAN). The percentage of ammonia in TAN can be calculated from water temperature and pH (Table 1).
Boyd, Percentage of un-ionized ammonia in waters, Table 1
| pH | Temperature (° C) 16 | Temperature (° C) 20 | Temperature (° C) 24 | Temperature (° C) 28 | Temperature (° C) 32 |
|---|
pH | Temperature (° C) 16 | Temperature (° C) 20 | Temperature (° C) 24 | Temperature (° C) 28 | Temperature (° C) 32 |
|---|---|---|---|---|---|
| 7.2 | 0.47 | 0.63 | 0.82 | 1.10 | 1.50 |
| 7.6 | 1.17 | 1.56 | 2.05 | 2.72 | 3.69 |
| 8.0 | 2.88 | 3.83 | 4.99 | 6.55 | 8.77 |
| 8.4 | 6.93 | 9.09 | 11.65 | 14.98 | 19.46 |
| 8.8 | 15.76 | 20.08 | 24.88 | 30.68 | 37.76 |
| 9.2 | 31.97 | 38.69 | 45.41 | 52.65 | 60.38 |
Ammonia is a metabolic waste that is potentially toxic to aquatic animals, but ammonium is essentially nontoxic at concentrations encountered in aquaculture systems. Ammonia toxicity is more likely at high feeding rates. The input of TAN and phosphate from feeding increases phytoplankton abundance. Phytoplankton photosynthesis causes pH to increase, which favors a higher percentage of ammonia.
Ammonium is absorbed by phytoplankton and other aquatic plants and converted to organic nitrogen in plant tissue. Plant uptake exerts a major control on TAN concentration, but when plants die and decay, ammonia is released. Nitrification, the process by which ammonia nitrogen is oxidized to nitrate by nitrifying bacteria, is an important control on TAN concentration.
For unexplained reasons, nitrite concentration sometimes increases in ponds. Nitrite also can be toxic to aquatic animals, for it is absorbed and interferes with the ability of blood to transport oxygen.
Denitrification
Denitrification is the process by which denitrifying bacteria in anaerobic sediment or water respire using oxygen from nitrate as a substitute for molecular oxygen. Nitrogen in nitrate is transformed to molecular nitrogen and diffuses into the atmosphere. Denitrification occurs through different pathways. Ammonia also is a gas that can diffuse from water to the air. A study at Auburn University found that diffusion of ammonia from aquaculture ponds ranged 0.01 to 1.00 kg nitrogen per hectare per day. Ammonia loss was most rapid on windy days.
A nitrogen “budget” was developed for channel catfish ponds at Auburn University. Feed accounted for 87.9 percent of the nitrogen input to ponds. Blue-green algae and bacteria do not fix nitrogen when ammonium and nitrate are present in ample amounts. Ponds usually had 1 or 2 mg per liter of plant-available nitrogen, which greatly limited nitrogen fixation. Of the nitrogen input to ponds, 31.5 percent was removed in fish, 17.4 percent was denitrified, 12.5 percent was lost by ammonia diffusion, and 22.6 percent accumulated in bottom soil organic matter.
C:N ratio and TAN
It is interesting to consider the effect of the carbon: nitrogen (C:N) ratio of organic matter on microbial activity. Organic matter with a C:N ratio of 15 or less usually decomposes quickly with the release of considerable ammonia. Organic matter with higher C:N ratios usually decomposes much slower with the release of little or no ammonia.
Microorganisms of decay remove TAN or nitrate from water to aid in decomposing residues with high C:N ratios. Because aquafeeds are rich in nitrogen and nitrogen fertilizers often are added to aquaculture ponds, organic matter tends to decompose rapidly and not accumulate to high concentrations in bottom soils.
For example, natural wetlands often contain plants of low nitrogen content. When the plants die, they decompose slowly and incompletely. Wetland soils can contain 40 percent or more organic matter. Conversely, in aquaculture ponds, organic matter concentrations in sediment seldom exceed 6 percent.
Typically, 2 to 8 kg nitrogen per hectare are applied to fertilized ponds at two- to four-week intervals. Phosphate fertilizer usually is added with nitrogen fertilizer. In freshwater, phosphorus rates should be about twice the nitrogen rates, but the opposite applies to ponds with brackish or sea water. This difference is probably related to a greater abundance of nitrogen fixers in freshwater. In shrimp farming, a sodium nitrate-based fertilizer can be used to encourage a high abundance of diatoms.
High TAN concentrations can occur in ponds where animals are offered manufactured feeds. Although ammonia seldom kills fish or shrimp, ammonia-nitrogen concentrations above 0.2 mg per liter can stress them and reduce growth. TAN concentrations above 2 mg per liter can result in potentially harmful ammonia levels in warm water when pH is above 8 (Table 1).
Water exchange can flush TAN from ponds, but this practice usually is discouraged, for it can spread diseases and favors larger pollution loads in effluents. Zeolite is not effective for removing TAN from pond waters, and the benefits of bacterial amendments and enzyme preparations for lowering TAN concentrations are questionable. Reduction in pond water pH through the application of commercial-grade sulfuric or hydrocholric acid to lessen the proportion of ammonia usually is not practical in large ponds.
Management
Improved feeds and feeding practices increase the proportion of nitrogen recovered in fish and shrimp and lessen the amount of ammonia excreted by the culture animals. Efficient aeration and water circulation that maintain adequate dissolved-oxygen concentrations in the water column and at the sediment-water interface enhance conversion of organic nitrogen to ammonia and oxidation of ammonia to nitrate by nitrifying bacteria.
Nitrite toxicity is sometimes a problem in freshwater fish culture. Increasing the chloride concentration through application of sodium chloride is a preventative measure used in channel catfish farming. Chloride interferes with nitrite uptake across the gills of the fish. Chloride concentrations about 20 times the nitrite concentration will prevent nitrite toxicity. For example, catfish farmers in Alabama, USA, try to maintain 50 to 100 mg per liter chloride in pond water as a preventative measure.
(Editor’s Note: This article was originally published in the March/April 2008 print edition of the Global Aquaculture Advocate.)
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Author
-

Claude E. Boyd, Ph.D.
Department of Fisheries and Allied Aquacultures
Auburn University
Auburn, Alabama 36849 USA
Tagged With
Related Posts

Responsibility
Ammonia nitrogen dynamics in aquaculture
The major sources of ammonia in aquaculture ponds are fertilizers and feeds, and problems with high ammonia are most common in feed-based aquaculture.

Responsibility
A look at unit processes in RAS systems
The ability to maintain adequate oxygen levels can be a limiting factor in carrying capacities for RAS. The amount of oxygen required is largely dictated by the feed rate and length of time waste solids remain within the systems.

Innovation & Investment
A review of unit processes in RAS systems
Since un-ionized ammonia-nitrogen and nitrite-nitrogen are toxic to most finfish, controlling their concentrations in culture tanks is a primary objective in the design of recirculating aquaculture systems.

Responsibility
Accuracy of custom water analyses varies
The reliability of trace element analyses reported by custom laboratories cannot be checked by simple techniques, and results may not always be accurate. One should check the reliability of major ion analyses by determining the charge balance and comparing the measured total ion concentration with the total ion concentration estimated from conductivity.

