Aquarium fish trade is one of Hawaii’s most valuable near-shore fisheries
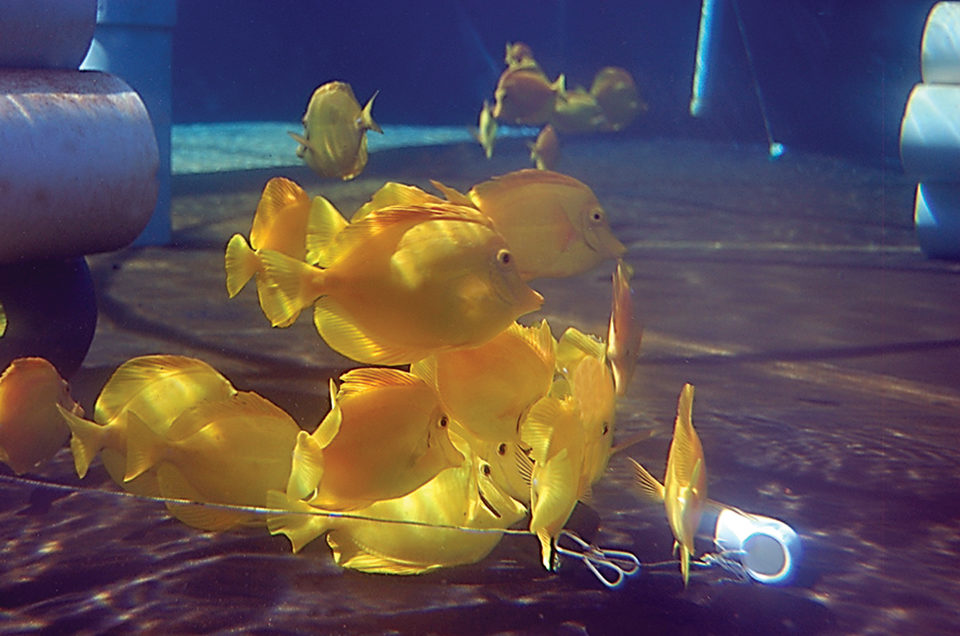
One of the premier visitor attractions in the U.S. state of Hawaii is its coral reefs, with over three million people coming to the state each year to experience them. In addition to their importance in recreation, Hawaii’s reefs are a source of livelihood for local fishermen.
The aquarium fish trade is one of the most valuable near-shore fisheries in Hawaii. Yellow tang, (Zebrasoma flavescens) make up more than 80 percent of the local aquarium fishery, with hundreds of thousands harvested annually to satisfy an ever-growing aquarium market. Currently, the sustainability of the collection of aquarium species, although regulated by the state Department of Land and Natural Resources, is being questioned by the local community. This is particularly evident on the west coast of Hawaii Island, which comprises more than two-thirds of the state’s aquarium fishery.
Captive rearing: conservation
In order to assist in the conservation of this important resource, the Oceanic Institute of Hawaii Pacific University is seeking to provide an alternative to the collection of these fish through the development of methods to culture them. The institute has been working for over a decade to solve the intricate problems associated with this remarkable challenge.
Recently, the authors have made significant progress toward establishing the first-ever captive-reared yellow tang. They now have a reliable source of eggs from conditioned broodstock and routinely get tens of thousands of larvae with which to work at a time. Prior research has enabled the larvae to be reared in large numbers through the first-feeding stages using copepod nauplii, but high mortality has often been observed in the days and weeks following.
Breakthrough
In an early 2014 yellow tang production run, however, something was different. After about day 14, when high attrition was usually seen in the tanks, a tank full of robust larval fish was observed. A single 1,000-liter larval-rearing tank yielded thousands of larvae out past the first three weeks.
The authors hadn’t had the opportunity to explore alternative feed items for the later-stage larvae. When copepods were short in supply, they added rotifers as prey, although the larvae did not appear to consume them. The larvae were also too small at this stage to consume artemia. Therefore, the larvae were provided additional cultured copepod nauplii as prey, with larger stages of nauplii and copepodites slowly added to the feeding mix.
At day 35, the larvae were moved to separate tanks to allow further exploration of alternative feeds and potential settlement cues like photoperiod and substrate. At this stage, the morphology of the larvae was very distinctive. The tang appeared nearly diamond-shaped, and most had already undergone flexion.
The authors were very encouraged at this point, since there were over 600 fish in the new tanks, and some could make it all the way to maturity. From day 35 to day 50, the fish developed rapidly, and there were noticeable changes in their morphology. Their bodies became deeper, and the heads and mouths became more pronounced. The dorsal and ventral spines began to recede, and the fish became more associated with the tank walls rather than swimming in mid-water. At this stage, the fish were about 1 cm in length and being fed a mixture of copepods of mixed sizes and newly hatched artemia nauplii.
Road block
At day 50, the fish appeared to hit a “road block.” Development slowed, and from days 50 to 60, the largest individuals exhibited heavy mortality. During this period, the authors routinely observed the striking inability of the larvae to consume artemia. The reduced development and mortality were likely due to the unsuitability of the prey type.
The larvae would routinely strike at and then miss the artemia, or sometimes successfully catch, but then spit out the prey. Larger copepods appeared to be too fast, and the larger copepodite or adult stages of Parvocalanus were also not successfully utilized by the later-stage yellow tang larvae.
With reports of wild yellow tang settling to the reef by day 60, it was clear the dietary and/or environmental requirements of the species were not being met. The authors nursed the last surviving individual, named Lucky, to day 83. Interestingly, some signs of the transition to settlement – further growth and morphology changes coupled with complete recession of spines – were seen just before day 82.
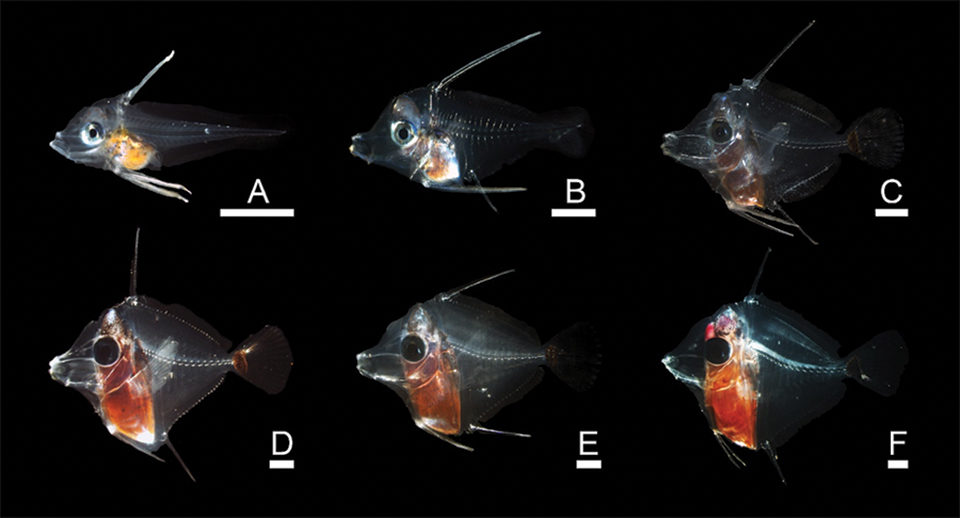
Scale bars = 1 mm. A = 14 days post-hatch (dph), B = 24 dph, C = 36 dph, D = 45 dph, E = 50 dph, F = 60 dph.
Minimal bacteria
While this run did not result in settled juvenile yellow tang, the advances were attributed to some changes in the early rearing protocols. The biggest difference was the fact that the larval rearing was done in clear water with no background algae using relatively high water turnover rates. Another important detail: Very little other activity was occurring in the hatchery during the run.
This resulted in the run coinciding with the system being started after several months of down time. Also, the tang larval tank was the sole recipient of water through the hatchery’s ultraviolet sterilizer, which was dosed at greater than 4,000 mJ per cubic centimeter.
The authors attribute much of the improved survival to the high water exchange utilizing very clean water, which reduced the bacteria load in the larval-rearing environment. These methods are currently being retested in conjunction with more focused attention on the bacteria associated with the live feeds.
Probiotics
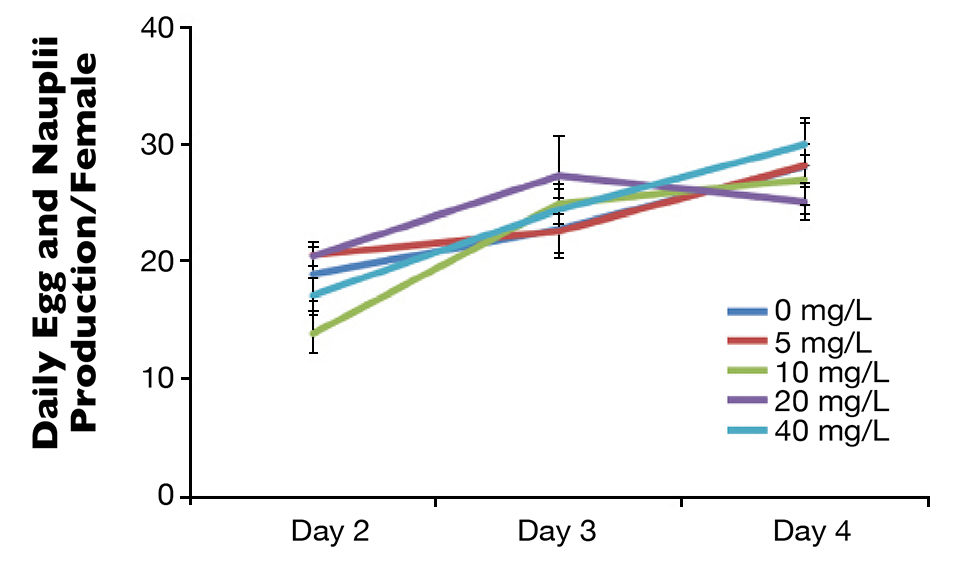
The authors are presently investigating the effects of probiotics as a potential means to improve the bacteria communities associated with the microalgae and copepod live feeds. Early examination of a commercial probiotic revealed no negative impact on copepod culture performance (Fig. 1), so it will be tested further at production scales. At the same time, bacteria that commonly occur in live feed cultures are being isolated using polymerase chain reaction amplification and DNA sequencing to identify species that may impact the larvae.
The goal of this work is to determine if the mortality often observed after first feeding can be correlated with the bacteria associated with the live prey and/or water in the larval tanks. Further, better understanding of the bacterial communities involved will help determine how probiotics might impact these communities.
(Editor’s Note: This article was originally published in the November/December 2014 print edition of the Global Aquaculture Advocate.)
Authors
-
Chatham K. Ferna, Ph.D.
Finfish Program
Oceanic Institute of Hawaii Pacific University
41-202 Kalanianaole Highway
Waimanalo, Hawaii 96795 USA -
Emma C. Forbes
Finfish Program
Oceanic Institute of Hawaii Pacific University -
M. Dean Kline
Finfish Program
Oceanic Institute of Hawaii Pacific University -
Shelby E. Allen
Finfish Program
Oceanic Institute of Hawaii Pacific University -
David J. Hoy
Finfish Program
Oceanic Institute of Hawaii Pacific University
Tagged With
Related Posts

Responsibility
Aquaculture gives endangered totoaba a fighting chance
The tenuous fate of a pint-sized porpoise, the critically endangered vaquita, is linked to a fish targeted by poachers fueling China’s appetite for maws. The vaquita remains in peril, but aquaculture presents some hope for the totoaba.

Health & Welfare
Biofloc technology holds potential for carnivorous fish species
Juvenile carnivorous African catfish performed well in biofloc-based systems, which could help produce better quality and more disease-resistant seed of this important aquaculture species and support the expansion of African catfish farming industry.

Innovation & Investment
Artemia, the ‘magic powder’ fueling a multi-billion-dollar industry
Artemia, microscopic brine shrimp used as feed in hatcheries, are the unsung heroes of aquaculture. Experts say artemia is still inspiring innovation more than 50 years after initial commercialization. These creatures are much more than Sea-Monkeys.

Intelligence
Off the Knife with Ned Bell, Vancouver Aquarium
We delve deeper into the foodservice industry’s engagement with sustainable seafood and aquaculture by talking to Ned Bell, the Vancouver Aquarium’s new executive chef. He continues to call on his Canadian culinary comrades to learn more about seafood, then help educate consumers.


