Mortality increased significantly under conditions of co-infection
Diseases are a major problem for tilapia farming. They cause high fish mortality, increase production costs and decrease profits. Parasites, bacteria and fungi are potential pathogens for tilapia.
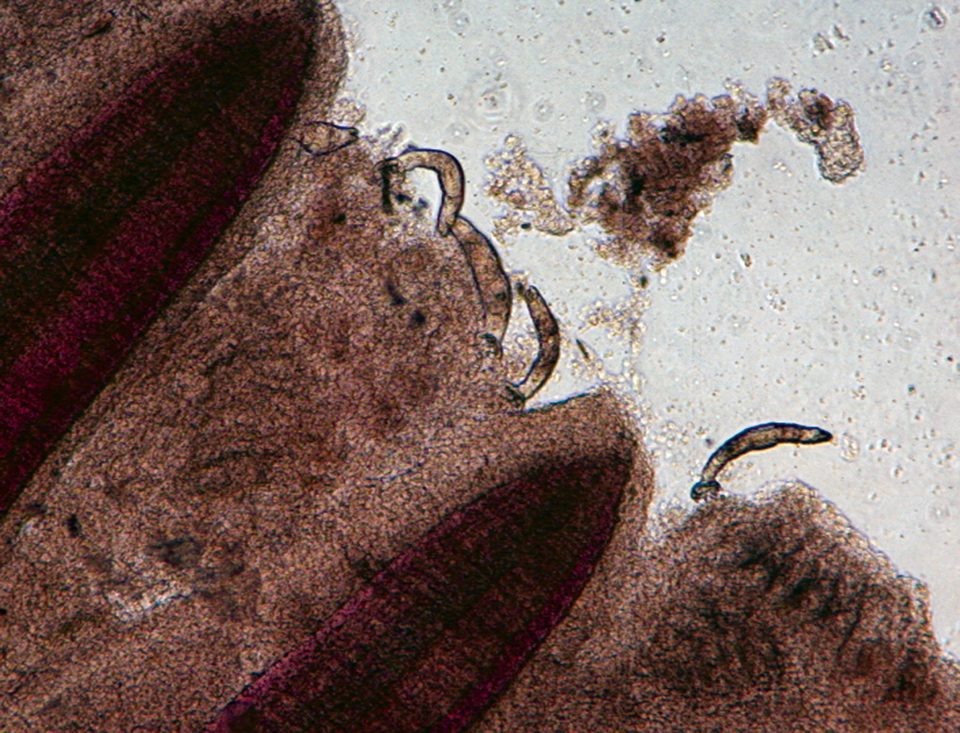
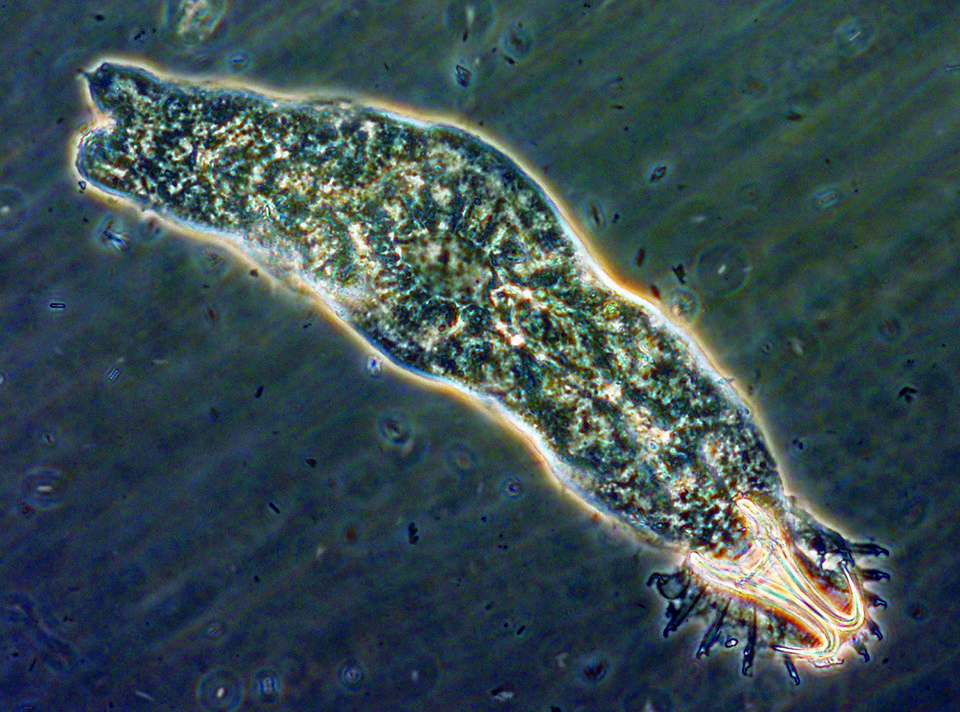
Gyrodactylus, for example, is a monogenetic trematode that appears on fish fins, body surfaces and gills, and has the ability to expand rapidly within a relatively short time due to high reproduction rates and direct transmission between fish. Streptococcus iniae is a severe bacterial pathogen that has caused production losses in most regions of the world where tilapia are cultured.
Since fish are constantly exposed to pathogens in water, tilapia can be co-infected with two or more pathogens. Both Gyrodactylus and S. iniae have been observed in tilapia from ponds, tanks and recirculating aquaculture systems. However, no study had been conducted to determine the association of the two pathogens in tilapia. The authors therefore conducted a study to evaluate whether tilapia infected with Gyrodactylus were more susceptible to S. iniae.
Streptococcus infection trial
The authors used Nile tilapia as experimental fish. After tilapia spawned in a pond, the fry were collected and reared in tanks using filtered, recirculated water. When the tilapia reached 9 cm in length and 12 g in weight, the fish were parasitized at an intensity of less than 10 Gyrodactylus per fish.
The tilapia were divided into two groups. In one group, the parasite intensity was allowed to increase by holding 50 or more fish in 57-liter aquariums without treatment for two weeks. The other group of tilapia was treated with formalin and potassium permanganate to eliminate parasite infection. After treatment, flowing water was provided, and the treated fish were allowed one week to recover.
Two hundred tilapia infected with Gyrodactylus and 200 fish free from the parasite were used in the infection trial. These fish were divided into four, 90-fish groups and received one of the following treatments: parasite infection and bacteria challenge, no parasite infection and bacteria challenge, parasite infection and no bacteria challenge, and no parasite or bacteria challenge. Each group was split evenly among three tanks.
The remaining fish – 20 infected with Gyrodactylus and 20 free of parasites – were examined for parasite load and bacterial infection prior to the trial. For fish challenged with S. iniae, the bacterial suspension was added to the tanks and fish exposed to the bacteria for one hour. The mortality of fish was recorded, and dead fish were examined for S. iniae daily for two weeks.
Results
The 20 Gyrodactylus-infected fish and 20 non-infected fish were cultured to verify their S. iniae-free status prior to the infection trial. None of the fish was positive for S. iniae prior to the trial.
Wet mount samples were prepared from fish fins and gills to assess parasite intensity under a microscope. The fish showed 20 Gyrodactylus in fins and 29 Gyrodactylus in gills in the group of fish with parasites prior to the S. iniae infection. No Gyrodactylus was detected in the group of fish free from the parasite.
Twelve buckets with aeration were each filled with 2 L of tank water and 30 fish. For fish exposed to the bacteria, S. iniae suspension was added, and fish were immersed in the water for one hour. The fish from each bucket were then moved to a 57-L aquarium with flowing water and aeration. Fish mortality was recorded, and dead fish were examined for bacterial infection daily for two weeks.
At the end of the infection trial, 38 of the 90 fish died in the group co-infected by Gyrodactylus and S. iniae, and only six were lost in the non-parasitized group challenged with S. iniae (Table 1). Tilapia co-infected by both parasites and bacteria showed 42.2 percent mortality, which was much higher than the 6.7 percent loss in the non-parasitized fish exposed to S. iniae challenge. No fish died in the group with only Gyrodactylus infection and in the group with no parasites or bacteria.
Xu, Mortality of Nile tilapia, Table 1
| Parasite Infection | Bacterial Challenge | Dead/Total Fish | Mortality (%) |
|---|---|---|---|
| Gyrodactylus | S. iniae | 38/90 | 42.2 |
| No parasite | S. iniae | 6/90 | 6.7 |
| Gyrodactylus | 0 | 0/90 | 0 |
| No parasite | 0 | 0/90 | 0 |
Bacterial samples from the dead tilapia were incubated on agar plates and identified with biochemical tests. Streptococcus iniae was isolated from 35 of 38 dead fish from the group infected by Gyrodactylus following bacteria challenge. S. iniae was also isolated from all six dead fish in the non-parasitized group exposed to bacterial challenge.
Perspectives
This research demonstrated that tilapia with a solo infection of Gyrodactylus or Streptococcus had less than 7 percent total mortality. However, the mortality increased significantly under conditions of co-infection. The results confirmed earlier impressions that under farm conditions, diseases from the pathogens can occur together with devastating results.
The invasion and movement of parasites from one location to another on fish cause mechanical injuries to the epitheliums of fish. These injuries can serve as entrances for bacterial invasion, making fish with Gyrodactylus infection more susceptible to Streptococcus.
Steps such as parasite quarantine, good water quality, reduction of fish density and early diagnosis can be used to prevent the parasite from entering culture facilities or building up in fish. The combined efforts of prevention and treatment not only reduce the direct damage by parasites, but also minimize fish mortality due to bacterial infection.
(Editor’s Note: This article was originally published in the March/April 2011 print edition of the Global Aquaculture Advocate.)
Authors
-
De-Hai Xu, Ph.D.
USDA Agricultural Research Service
Aquatic Animal Health Research Laboratory
990 Wire Road
Auburn, Alabama 36832 USA[118,111,103,46,97,100,115,117,46,115,114,97,64,117,120,46,105,97,104,101,100]
-
Craig Shoemaker, Ph.D.
USDA Agricultural Research Service
Aquatic Animal Health Research Laboratory
990 Wire Road
Auburn, Alabama 36832 USA -
Phillip Klesius, Ph.D.
USDA Agricultural Research Service
Aquatic Animal Health Research Laboratory
990 Wire Road
Auburn, Alabama 36832 USA
Tagged With
Related Posts

Health & Welfare
A holistic management approach to EMS
Early Mortality Syndrome has devastated farmed shrimp in Asia and Latin America. With better understanding of the pathogen and the development and improvement of novel strategies, shrimp farmers are now able to better manage the disease.

Health & Welfare
A study of Zoea-2 Syndrome in hatcheries in India, part 2
Indian shrimp hatcheries have experienced larval mortality in the zoea-2 stage, with molt deterioration and resulting in heavy mortality. Authors considered biotic and abiotic factors. Part 2 describes results of their study.

Health & Welfare
A study of Zoea-2 Syndrome in hatcheries in India, part 3
In this third and final part, authors present recommendations to help reduce the incidence of Zoea-2 Syndrome, which is not caused by any known infectious agents in P. vannamei hatcheries in India.

Health & Welfare
AHPN inferences based on behavior of vibrio bacteria
Vibrio parahaemolyticus, a strain of which is the cause of acute hepatopancreatic necrosis (AHPN), has both virulent and benign strains. This strain colonizes the stomachs of shrimp by the formation of a biofilm, which protects it from antibiotics and other potential treatments.


