Biofilms can spread antimicrobial-resistance genes

In their effort to maintain vitality, microorganisms seek solid surfaces conditioned with sufficient nutrients for growth. As they grow and multiply, the newly formed cells cling to each other as well as the surfaces, forming a confluently growing colony of microorganisms. When this mass of cells becomes large enough to entrap debris, nutrients, and other microorganisms, a microbial biofilm is established.
Microbial biofilms
In simple terms, a biofilm is a community of interacting microbes embedded in an organic polymer matrix adhered to a surface. The matrix of a biofilm consists largely of water, with the remainder composed of an assortment of polysaccharide and glycoprotein polymers often referred to as extracellular polymeric substances (EPS).
The microorganisms are not uniformly distributed in the biofilm. The colonization of a surface by one bacterial genus often influences the attachment of other genera to the same surface. For example, biofilm formation by Listeria monocytogenes can be enhanced in the presence of Pseudomonas fragi.
Bacteria gain a number of advantages from living in biofilms. For example, the microbial cells are to some extent protected from the outside environment. Biofilms are resistant to antibacterial agents, particularly disinfectants, and heat. They serve as a reservoir for many species of bacteria that would otherwise be unable to resist environmental fluctuations. Also, the microorganisms in biofilms can be dynamic, changing their composition due to the presence of other microorganisms in the environment.
Useful characteristics
Although biofilms are often considered in a negative sense, their characteristics can, on occasion, be exploited. Their organic nutrient-trapping capabilities are successfully used in wastewater treatment to reduce organic content before release.
Biofilms are the preferred method for biological nitrification or denitrification of wastewater. Nitrification is carried out in two stages by the autotrophic bacteria Nitrosomonas and Nitrobacter. It can be achieved within any aerobic biological treatment method, such as the suspended growth, activated sludge process; attached (supported) growth, trickling filter process; or packed bed process. The use of rotating biological contactors can also achieve nitrification.
Rotating biological contactors are used in many recycling aquaculture production systems. In these systems, differences in the attachment properties between the nitrifying and nonnitrifying biofilms would be expected. Biofilm thickness in rotating contactors is influenced by disc rotation speeds, substrate concentration in the bulk liquid, and temperature.
Economic, health hazards
Biofilms in man-made environments can present economic and health hazards. They pose important problems for industry that result in decreased productivity and replacement of expensive equipment. For example, mixed-species biofilms containing sulfur-reducing and oxidizing bacteria can corrode metal surfaces and lead to increased flow resistance and equipment blockages. Increased accumulations of particles on surfaces can result in corrosion due to cathodic depolarization and acid production.
Biofilms are responsible for the fouling and corrosion of solid surfaces submerged in water, including those of heat exchangers and water distribution systems. The most evident problem caused by biofilms in heat exchanger systems is a decrease in heat transfer that can reduce efficiency by over 90 percent. The dangerous Legionella pneumophila, which causes Legionnaires disease, has also been isolated in biofilms from water connected to air conditioners.
Modern food processing supports biofilm-forming bacteria on food contact surfaces due to the mass production of products, lengthy production cycles, and vast surface areas. Biofilms have been found in various food-processing facilities, including processors of raw, cooked, fermented, and smoked fish, mussels, shrimp, and crabs.
Once biofilms form on food-processing surfaces, they are difficult to remove, often resulting in persistent and endemic populations. For example, the strain persistence of Listeria has been documented to last from eight months to 10 years due to resistance to cleaning and sanitizing regimes. Biofilm formation by pathogenic bacteria on food contact surfaces can lead to contamination of food products during processing, which lowers product shelf life or results in human foodborne illness, both of which can result in economic loss.
Recirculating aquaculture systems can be also be adversely affected by biofilms. Many potential pathogens, such as Mycobacterium, Staphylococcus, enteroviruses, mycoplasmas and protozoa, have been associated with the biofilms.
Biofilm removal
It is not practical to clean and sanitize production and processing systems frequently enough to prevent the attachment of cells to surfaces, as cell attachment can occur within a few minutes or hours. The removal of biofilms during cleaning is significantly enhanced by applying mechanical force to surfaces, such as by using high-pressure sprayers capable of pressures greater than 250 pounds per square inch at a distance of 125-250 mm from the surface, as well as scrubbing pads or brushes.
It is important to break up and remove the EPS matrix. If the EPS matrix is not completely removed when cleaning and sanitizing a surface, bacteria will more readily reattach to the surface and a biofilm will form again, even if the previous bacteria were destroyed.
Cleaning procedures should not produce aerosols, which can speed the proliferation of bacteria as they contaminate other equipment and portions of the facility. Foaming cleaners and sanitizers do not produce aerosols, and some have excellent detergent properties.
Some reports have indicated that the use of chemical sanitizers can exert a selective pressure on attached populations that results in the emergence of resistant strains. Furthermore, the prevalence of antibiotic-resistant strains has steadily increased in recent times. Such resistant strains have been shown to exhibit cross-resistance to other antimicrobial agents, such as sanitizers.
The human pathogen Staphylococcus aureus, which has resistance to penicillin, also exhibits resistance to sanitizers that contain quaternary ammonium chloride. It has been shown that bacteria in a biofilm can also transfer antibiotic/sanitizer resistance to other members of the population.
Bacteria control
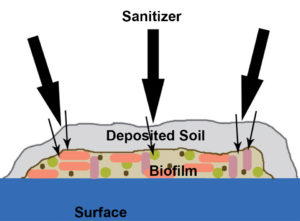
Biofilms can also represent a reservoir for the spread of antimicrobial-resistance genes. One major problem in controlling bacterial populations is that antibiotics or biocides are most often tested against free-living or free-floating planktonic cultures, usually in pure culture. In reality, they should be tested against multispecies cultures and biofilm communities.
One study showed that microorganisms in biofilms were 150-3,000 times more resistant to hypochlorous acid and 2-100 times more resistant to monochloramine than were unattached cells. Sodium hypochlorite is the oldest and most widely used of the chlorine compounds employed in chemical disinfection.
Care must be taken to make sure that sufficient free chlorine is available in sanitizing solutions, since chlorine reacts competitively with organic material to reduce the concentrations of the sanitizers that reach the bacteria. In general, chlorine dioxide, peracetic acid, and acidic quaternary ammonium were the most effective sanitizers on attached cells.
There have been several evaluations of biofilms’ strength of attachment and growth rates on different surfaces. For example, biofilms consisting of Escherichia coli grew better on polypropylene than stainless steel, while they did not grow on glass. Biofilms have been shown to preferentially attach and grow in surface cracks, crevices, dead ends, corners, gaskets, joints, and valves, particularly globe valves.
(Editor’s Note: This article was originally published in the July/August 2007 print edition of the Global Aquaculture Advocate.)
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Author
-

George J. Flick, Jr., Ph.D.
Food Science and Technology Department
Virginia Tech/Virginia Sea Grant (0418)
Blacksburg, Virginia 24061 USA[117,100,101,46,116,118,64,103,107,99,105,108,102]
Tagged With
Related Posts

Responsibility
Importance of aeration intensity for nitrification in biofilms in intensive biofloc systems
This study evaluated the importance of aeration intensity for the nitrification process in biofilm in intensive biofloc technology aquaculture systems.

Health & Welfare
Nitrifying biofilms critical for water quality in intensive shrimp RAS
Nitrifying bacteria readily form biofilms on surfaces, and colonization by these important bacteria on the interior walls of RAS production units likely provides an additional source of nitrification.

Health & Welfare
AHPN inferences based on behavior of vibrio bacteria
Vibrio parahaemolyticus, a strain of which is the cause of acute hepatopancreatic necrosis (AHPN), has both virulent and benign strains. This strain colonizes the stomachs of shrimp by the formation of a biofilm, which protects it from antibiotics and other potential treatments.

Health & Welfare
Antibiotic-resistant bacteria, part 2
The development of antimicrobial resistance genes in human pathogens as a consequence of exposure to antibiotics in aquaculture is widely documented. Reports implicate foodborne antibacterial-resistant pathogenic bacteria in human disease.

