Potential alternative to antibiotics being evaluated to prevent vibriosis in crustaceans
For several years, antibiotics have been successfully used to control pathogenic bacteria in aquaculture. However, during the last decade, health and environmental agencies have identified negative effects from antibiotics and imposed restrictions on their use. Phage therapy – the use of bacteriophages, viruses that kill bacteria – is one emerging method for controlling pathogenic bacteria.
Bacteriophages
Bacteriophages are the most numerous organisms on earth – around 100 billion viral particles can be found in a liter of water. They play a critical role in ocean dynamics by daily removing 20 percent to 40 percent of the total bacterial populations on earth. Phages have a restricted range of host bacteria, and therefore infection by a particular phage does not act on total microbial assemblage but rather on specific sub-populations.
Since their discovery in 1917, phages have been used for therapy and biocontrol of pathogenic bacteria. Successful treatments were developed to treat infections in humans and livestock, but they were somewhat forgotten when antibiotics were widely commercialized.
Phage therapy in aquaculture
In aquaculture, phage therapy was first evaluated in the early 1980s to control aeromonad septicemia and Edwardsiellosis in loaches, and later to control vibriosis in milkfish, as well as problems in other species.
Currently, this therapy is being evaluated to prevent vibriosis in crustaceans, Streptcoccus iniae in barramundi and Aeromonas hydrophila in striped sea bass. Most studies have been carried out with juvenile or adult finfish, where the phages were injected or administered with food. Phage therapy will be more important in fish hatcheries or the culture of invertebrate species, where vaccination is currently not possible.
Better than antibiotics?
Phage therapy is highly specific and offers quick bactericidal effects. The self-replicating phages are stable and have low toxicity and a low cost of production, as well (Table 1).
Diaz, Advantages and disadvantages of the use of phages, Table 1
| Characteristic | Practical Advantages | Potential Problems |
|---|
Characteristic | Practical Advantages | Potential Problems |
|---|---|---|
| High specificity | No detrimental effect on beneficial bacteria or biologic filtration systems. Compatible with probiotics and bioremediation bacteria. | Pathogenic bacteria must be clearly identified. Treatment is ineffective on mixed infections. |
| High stability | Lingering protection. | Stability could be affected during the use of some chemical substances. |
| Quick bactericidal effect | Destroy target bacteria – including antibiotic-resistant strains – in minutes. | Effects could be affected by degree of disease progression or accessibility of target bacteria. |
| Self-replicating, self-limiting | Long-term effect and low dose requirement. | Persistence in aquaculture systems has not been evaluated. |
| No toxic or allergenic effects to humans, animals or plants | Safe to use in aquaculture. | No evaluations of interactions between lytic and lysogenic phages have been completed in terms of the exchange of genes and potential risks. |
| Low cost | Less expensive than antibiotics. | |
| Naturally abundant and diverse in the environment | Large sources for new isolations. | Further research on phage effects in aquaculture systems is needed. |
These attributes make phage therapy a very attractive technology for aquaculture. Also, considering that phages are authorized by the Food and Drug Administration in the United States to prevent bacterial contamination in human foods, their use will likely find few legal restrictions. The technology is now close to being commercially applied.
Research with larvae of black tiger shrimp, Penaeus monodon, showed that the use of Vibrio harveyi phages improved larval survival by almost double that recorded for shrimp treated with a mixture of antibiotics. These results are very encouraging, but reproducibility would depend on the particular conditions in each hatchery. Most phages only infect one kind of bacteria, so if bacteria other than the target species are implicated in mortality, phage therapy will not work. One possibility could be the use of mixtures of phages.
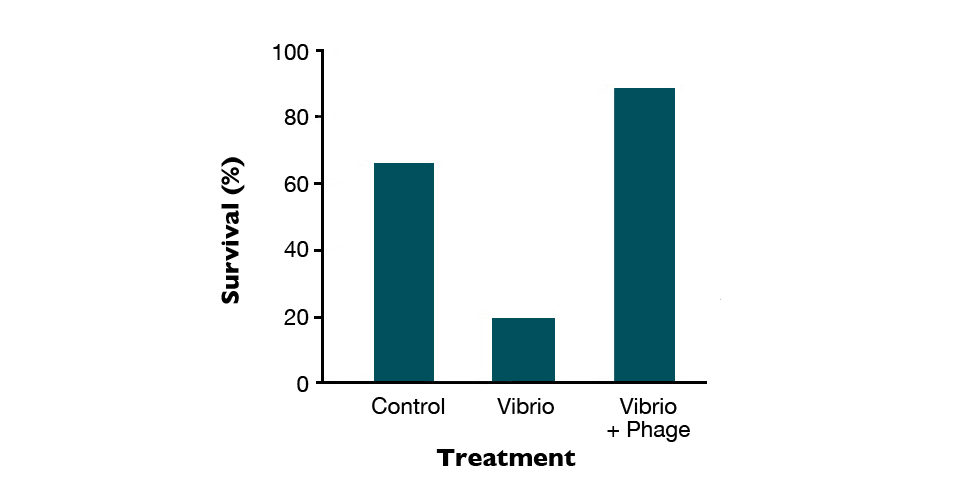
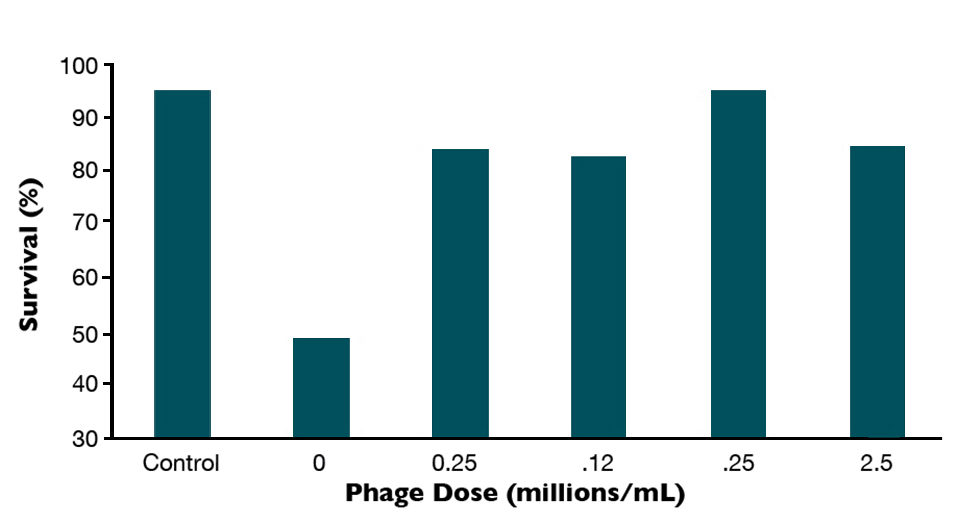
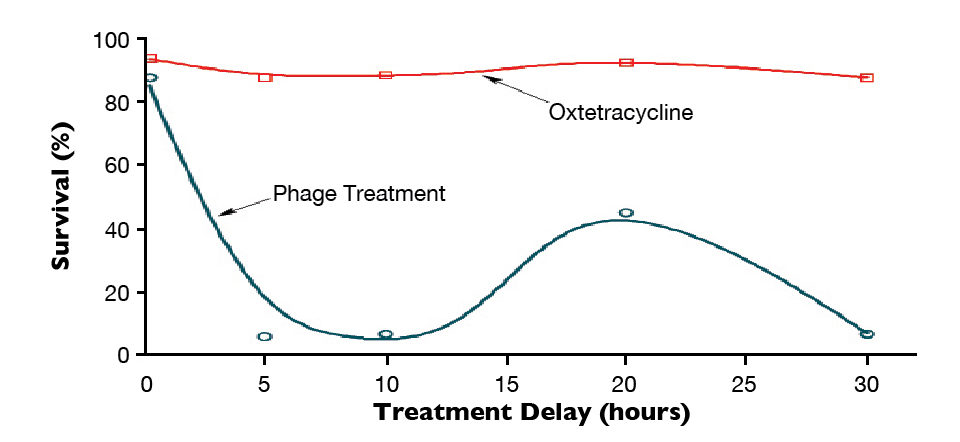
In a recent study with artemia challenged with V. parahaemolyticus, phage therapy effectively reduced mortality caused by the bacteria (Fig. 1), even when the dosage of phages was critically reduced (Fig. 2). Apparently, their beneficial effects depend on the degree of disease progression. The mortality of Artemia was completely controlled when the phages were added at the early stages of infection, while in other cases, the results were variable (Fig. 3). By contrast, in the same survey, the antibotic oxytetracycline was not affected by the degree of disease progression.
Challenges
Current research is evaluating the phage-coded enzymes that destroy bacterial cell walls or the proteins that use the phages to control bacterial metabolism. The success of phage therapy depends on the ability of phages to reach the target bacteria, so if the bacteria penetrate tissues or settle down in biofilms, it is more difficult to access them, and phage therapy will not work.
In addition, a phage mixture that is effective to treat infections in a region may not be effective for treating the same infection elsewhere. There are two explanations for this failure. The pathogenic bacteria from different places could have different patterns of susceptibility to phages, or different species of pathogenic bacteria could be implicated. For phage therapy, it is important to know the specific pathogenic bacteria in order to select the specific phage treatment. This does not occur with antibiotics, because in general they have a broad spectrum of effect.
For most shellfish, bacteriological diagnostics traditionally consist of the use of plating media to evaluate the abundance of pathogenic vibrios in culture environments. This becomes more complicated when the ability of the vibrios to induce mortality is less defined. In most cases, synergic interactions among the microbial community instead of a single pathogen probably cause the problems, making it difficult to reduce animal mortality by using phage therapy.
For example, phage therapy was ineffective in treating furunculosis in Atlantic salmon, but factors such as the growth of resistant bacteria or the effects of immune systems to inactivate the phages were implicated. In some instances, the use of phages in aquaculture will probably be considered a preemptive biocontrol treatment instead of corrective therapy.
Other aspects that need attention include the legalities regarding the use of phages, the effects of production conditions involving ozone, ultraviolet light, formalin or chlorine, and the risk of phage-to-phage transmission of undesirable genes during a co-infection.
(Editor’s Note: This article was originally published in the January/February 2010 print edition of the Global Aquaculture Advocate.)
Author
-
Sergio F. Martínez Díaz, Ph.D.
Microbiology and Molecular Biology Laboratory
Technology Development Department
Interdisciplinary Center of Marine Science
Playa el Conchalito S.N.
La Paz, Baja California Sur, Mexico
Tagged With
Related Posts

Health & Welfare
A holistic management approach to EMS
Early Mortality Syndrome has devastated farmed shrimp in Asia and Latin America. With better understanding of the pathogen and the development and improvement of novel strategies, shrimp farmers are now able to better manage the disease.

Health & Welfare
Aiding gut health with a natural growth promotor
A study with Nile tilapia conducted in commercial production cages in Brazil showed the potential – in the absence of major disease threats – of a commercial, natural growth promotor that modulates the microbiota (inhibiting growth of pathogenic bacteria and promoting growth of beneficial bacteria) and inhibits quorum sensing.

Health & Welfare
Potential applications of bacteriophages for AHPND control
Study demonstrates that isolated phages tested are effective in controlling AHPND infection in farmed penaeid shrimp and inhibiting bacterial growth.

Innovation & Investment
Scottish firm honing bacteriophages into aquaculture-disease assassins
Scottish biotech firm Fixed Phage aims to bottle the powers of bacteriophages to deploy these “bacteria killers” on some of the world’s most destructive aquaculture diseases.


