If antivirus bacteria dominate the water environment, virus transfer among fish communities may be repressed

days was added to seawater containing striped jack larvae.
Several viral diseases have seriously affected the aquaculture industry worldwide. Major examples of affected species include farmed (Penaeus monodon) in Taiwan, where production went from about 90,000 metric tons (MT) in 1987 to 20,000 MT in 1989, and has still not recovered. In Japan, the P. japonicus farming industry has been affected by WSSV since 1993, and many nursery ponds in the western part of Japan stopped production. More recently, WSSV devastated the shrimp farming industry in Latin America.
Other notable cases include Infectious Pancreatic Necrosis Virus (IPNV) which infects salmon; Hirame Rhabdovirus (HIRRV), which infects flounders; the Yellowtail Ascites Virus (YAV) which infects yellowtail; Striped Jack Nervous Necrosis Virus (SJNNV), which mainly infects striped jack; the Irido Virus, which infects sea bream; and many more – all of which cause serious damage in aquaculture.
Viral infections
There are three types of viral infection. The first is lytic infection, where the virus attaches to a host cell and injects its nucleic acid, causing the host cell to produce numerous progeny viruses. The cell then bursts and releases the progeny, beginning the cycle again. The second type is chronic infection, where the release of progeny virus from the host cell is non-lethal and occurs by extrusion or budding over several generations.
The third type of viral infection is lysogeny, where after injection, the viral genome becomes part of the genome of the host cell and reproduces with the genetic material in the host cell line, unless a switch to lytic infection is induced. Induction is commonly caused by DNA damage, such as that caused by ultraviolet light or agents like mitomycin C.
Viruses in marine environments
Viruses are very abundant in marine plankton. As obligate parasites of cellular organisms, the viruses are usually specific to certain hosts. Viruses infect bacteria, cyanobacteria and protists, although it appears most of the viruses present in seawater infect bacteria and are responsible for 10 to 40 percent of total bacterial mortality.
In addition, the release of dissolved organic matter during lysis of microbes is thought to stimulate bacterial activity at the expense of large organisms. It can also lead to increased retention of nutrients in the uppermost layer of the water column that receives sufficient light for photosynthesis. Other important roles of viruses include genetic transfer and influence on species composition.
Abundance
Using the direct counting method with electron microscopy, recently reported virus counts ranged 104 to 108 virus particles per ml. These numbers were up to 107 times higher than previous reports of virus particle numbers in natural aquatic environments, which were based on counts of plaque-forming units using various host bacteria. The wide range of viral concentrations indicates the possible effect of anti-viral microorganisms on the presence of virus particles in seawater. This helps explain why the concentration of viruses fluctuates so greatly in seawater and fresh water.
In addition, viruses have the ability to transfer from one infected organism to another through the water environment. If antivirus bacteria dominate the water environment, virus transfer among fish communities may be repressed to a large extent. Basednon this concept, antivirus bacteria are used in larval-rearing procedures in practical aquaculture applications.
Determination of antivirus activity
According to one hypothesis, bacteria that are able to repress the growth of other bacteria may also inhibit the growth of viruses. Therefore, as described by Maeda (1999), the first step in finding antivirus bacteria in seawater should be to determine antibacterial activity. This procedure is easier than determining antiviral activity directly. Therefore, checking for antiviral activity might not always be necessary for those who want to avoid the complicated procedures.
Maeda, Index* of IHNV damage to total cell** area, Table 1
| Incubation Period (day) | Incubation Period (day) | Incubation Period (day) | Incubation Period (day) | Incubation Period (day) | Incubation Period (day) | Incubation Period (day) | Incubation Period (day) |
|---|
Incubation Period (day) | Incubation Period (day) | Incubation Period (day) | Incubation Period (day) | Incubation Period (day) | Incubation Period (day) | Incubation Period (day) | Incubation Period (day) | |
|---|---|---|---|---|---|---|---|---|
| Experiments | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Reference | 0 | 0 | +1 | +1 | +2 | +3 | +4 | +5 |
| Bacterium (VKM-124) | 0 | 0 | +0 | +1 | +1 | +1 | +3 | +4 |
* Ratio (area) of cytopathic cells to the total cell area examined.
+1: <10%, +2: 10~25%, +3: 25~50%, +4: 50~75%, +5: >75%
** Epithelioma papillosum cyprini cells.
Table 1. Index* of IHNV damage to total cell** area in fraction treated with probiotic bacterium and reference fraction.
Using anti-virus bacteria for rearing fish larvae
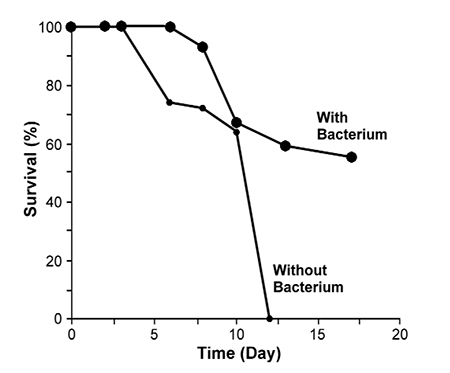
An example is the bacterial strain VKM-124 (Pseudoalteromonas undina), which has a Vibrio-static activity (Maeda, 1999) and is used in aquaculture practice. The presence of this bacteria delayed the onset of IHNV symptoms by about two days (Table 1).
This strain – which shows a strong antibacterial activity – can repress the growth of the virus. VKM-124 shows the ability to prevent fish larvae from being infected by SJNNV, Baculo-like viruses and Irido virus. When this useful strain was added to water at a concentration of about 106 cells per milliliter in larvae-rearing containers of penaeid shrimp, striped jack and sea bream, the survival rates of these larvae were much higher with the bacterium than without it. All fish larvae died due to viral disease without the addition of the bacterium.
Possible role of enzymes
The outer part of a virus particle comprises a layer of coating proteins that assemble to form the capsid, which facilitates the attachment, and perhaps penetration, of the virus upon contact with new susceptible cells. Aranishi (1999) reported the proteases present at the surface of the Japanese eel (Anguilla japonica) degrade the cell wall of many kinds of pathogenic bacteria.
He suggested that these proteases, epidermal cathepsins L and B, act as part of the nonspecific defense of the eels. This result suggests that proteases and other enzymes, including chitinase produced by probiotic microorganisms resident in the surface layer of the fish, block entry of pathogenic viruses by degrading viral coat proteins.
Conclusion
Viruses in seawater spread from infected fish to those not yet suffering from disease. However, probiotic bacterium may act to inhibit the migration of viruses among fishes. In addition, if fish feed on these probiotic bacteria, it could help to strengthen their immune systems, as was shown in the promotion of immune activities of vertebrates with bacteria. With such useful effects and features, probiotic bacteria could prove effective in protecting fish from the spread of viral diseases in aquaculture.
Note: Cited references are available from the author.
(Editor’s Note: This article was originally published in the February 2001 print edition of the Global Aquaculture Advocate.)
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Author
-
Masachika Maeda, Ph.D.
Director of Fisheries Division
Japan International Research Center for Agricultural Sciences
Ministry of Agriculture, Forestry and Fisheries
Tsukuba, Japan
Tagged With
Related Posts

Health & Welfare
A holistic management approach to EMS
Early Mortality Syndrome has devastated farmed shrimp in Asia and Latin America. With better understanding of the pathogen and the development and improvement of novel strategies, shrimp farmers are now able to better manage the disease.

Health & Welfare
‘Big picture’ connects shrimp disease, inbreeding
Disease problems on shrimp farms may be partly driven by an interaction between management practices that cause inbreeding in small hatcheries and the amplification by inbreeding of susceptibility to disease and environmental stresses.

Health & Welfare
Caveat emptor when seeking for EMS solutions
The development of polymerase chain reaction testing to detect the bacteria that cause EMS is important, but confirmation by bioassay of presumptive positives to ensure pathogenicity is a prudent intermediate step.

Health & Welfare
Genome editing: Potential to improve aquaculture breeding, production, Part 1
Due to high fecundity and external fertilization, most aquaculture species are amenable to genetic improvement technologies, including genome editing.


