SELDI-TOFMS a novel, cost-efficient technology

The development of rapid and sensitive tools to assess the health of farmed fish would be of great benefit to the aquaculture industry. Current methods to assess fish include such techniques as challenge tests and immunological and histological measurements. These methods can be very time-consuming and costly.
Recent research by the authors evaluated the use of surface-enhanced laser desorption per ionization time of flight mass spectrometry (SELDI-TOFMS) to identify peptides or proteins with significantly altered expression levels in treatment and control group samples. This novel, cost-efficient technology has the capacity to accurately and economically assess the welfare of farmed fish. The next step in developing a new tool to assess fish condition would be the identification of marker proteins to develop antibody-based assays.
Research setup
SELDI-TOFMS is a high-throughput technique that has been used in the field of medicine to identify disease-specific biomarkers. The technology consists of a protein chip array, the mass spectrometer, and the software to analyze the spectrums. Due to the specific binding capacity of the protein chip array, a subset of proteins from the samples is presented to the spectrometer.
In an initial study to ascertain the effects of diet on the protein profiles of fish, Atlantic salmon of 150 g initial weight were fed over three months a control diet containing 100 percent marine components, a diet with 100 percent vegetarian components, or a diet with 10 percent marine components. Samples were taken from the salmon to look for diet-specific alterations in the plasma and mucus protein profiles.
In a second study, Atlantic salmon with a mean weight of 160 g were kept in sea water tanks at densities of 100, 200, or 300 kilograms per m3 for six hours. Control fish were kept at 22 kilograms per m3. After the stress period, the density of all tanks was returned to 22 kilograms per m3.
Samples of plasma and mucus were taken immediately at the cessation of stress and then at intervals of 60, 120, 180, 360, and 1,440 minutes throughout the poststress period. Seven to 10 fish were sampled from each tank at each time point.
Mucus, plasma sampling
Standardization of the technique used for obtaining mucus samples from fish is an important prerequisite for successful protein profiling. Sampling was performed using a metal spatula cleaned with ethanol, which was scraped over both sides of the fish. Areas of discolored mucus were avoided.
Prior to analysis, mucus samples were centrifuged to remove scales and cell debris, and diluted with a preparation buffer. Both mucus and plasma samples were then diluted in binding buffer before being applied to the protein chip arrays. The prepared arrays were then placed in the protein chip reader.
Following irradiation of the samples with the laser and collection of the released ions by the detector, a mass spectrum is produced that consists of the mass:charge ratios (milligrams per zinc) together with the corresponding intensities of the component proteins. Examples of spectrums from mucus and plasma samples are shown in Fig. 1.
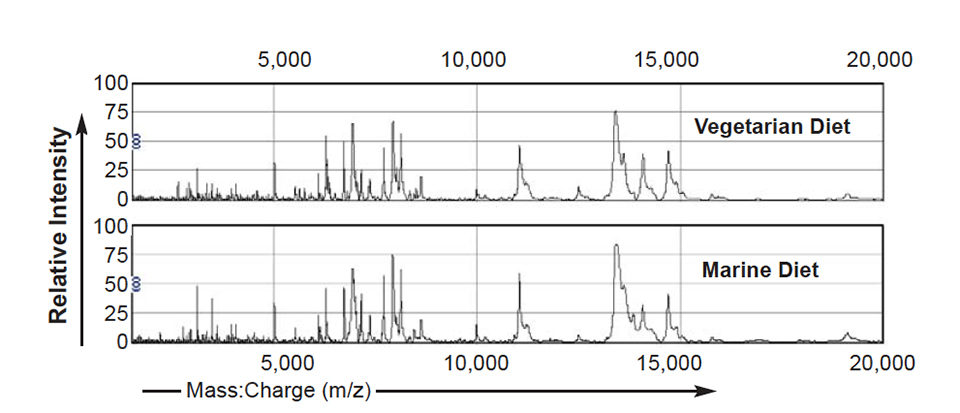
Results
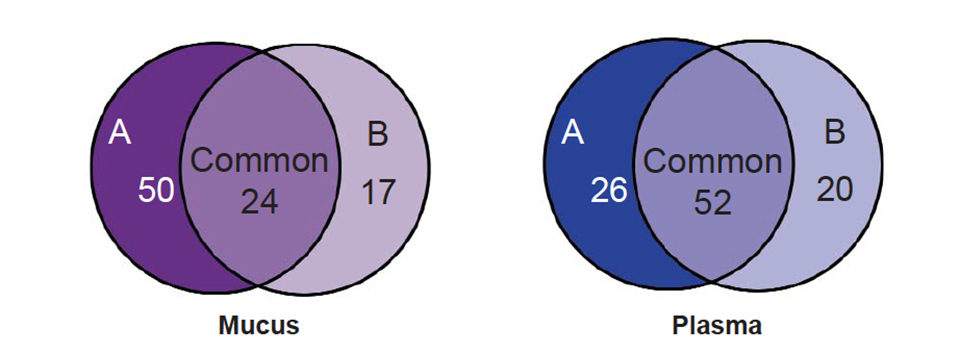
Various statistical methods can be used to analyze these spectrums. Using a univariate approach such as the Mann-Whitney T-test, lists of proteins differentially expressed in treatment groups are compared to controls. In a summary of these results, Fig. 2 shows that when comparing the spectrums from fish fed diet A or B to the control diet C, 50 diet-specific peaks were significantly altered in intensity (P < 0.05) in spectrums from fish fed diet A (100 percent vegetarian diet), whereas only 17 diet-specific peaks were significantly altered in intensity in spectrums from fish fed diet B (10 percent marine diet).
Twenty-four significant peak alterations common to both treatment diets were identified in the mucus spectrums. For the plasma spectrums, 26 peaks were significantly altered in the 100 percent vegetarian diet compared to the control diet, whereas 20 peaks were significantly altered in spectrums from the fish fed the 10 percent marine diet. Finally, 52 peaks were significantly altered in spectrums from fish fed either diet A or diet B.
In a more sophisticated statistical approach, multivariate analysis in the form of multidimensional scaling (MDS) was performed to identify patterns of proteins or single proteins to discriminate between treatment groups.
The MDS plots showed segregation of the data according to the diet the fish received. In Fig. 2, the data shown were produced by taking the means of peak intensities of corresponding peaks across 10 spectrums from fish fed one diet. Further analysis determined that the difference in intensity of two peaks alone contributed to the segregation of these groups. For plasma, the two peaks allowing this segregation had mass:charge ratios (m/z) of 2,649.8 and 3,521.3, and for mucus m/z 3,600.8 and 7,941.8.
In the second study, where the effects of density stress on protein profiles the means of peak intensities of corresponding peaks across 10 spectrums from fish fed one diet. Further analysis determined that the difference in intensity of two peaks alone contributed to the segregation of these groups. For plasma, the two peaks allowing this segregation had mass:charge ratios (m/z) of 2,649.8 and 3,521.3, and for mucus m/z 3,600.8 and 7,941.8.
In the second study, where the effects of density stress on protein profiles were examined, density-dependent alterations in the profiles were detected. These differences resulted in the segregation of the data according to the density stress to which the fish had been subjected prior to the poststress period during which samples were taken.
Further research
Protein profiling can provide sets of proteins specifically altered by diet or density stress. This information can be used as a diagnostic tool in itself, with the proteins acting as condition-specific markers. However, further work involving identification of the amino acid sequences of the set of proteins would allow us to develop antibody-based tests to measure alterations in specific protein concentrations.
(Editor’s Note: This article was originally published in the September/October 2006 print edition of the Global Aquaculture Advocate.)
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Authors
-
Fiona Provan
International Research Institute of Stavanger
Mekjarvik 12, N- 4070
Randaberg, Norway[111,110,46,104,99,114,97,101,115,101,114,115,105,114,105,64,110,97,118,111,114,112,46,97,110,111,105,102]
-
Odd Ketil Andersen
International Research Institute of Stavanger
Mekjarvik 12, N- 4070
Randaberg, Norway -
Shaw Bamber
International Research Institute of Stavanger
Mekjarvik 12, N- 4070
Randaberg, Norway -
Ramon Fontanillas
Nutreco Aquaculture Research Centre
Stavanger, Norway -
Wolfgang Koppe
Nutreco Aquaculture Research Centre
Stavanger, Norway
Tagged With
Related Posts

Aquafeeds
A new nutrient for aquaculture, from microbes that consume carbon waste
Biotechnology firm NovoNutrients aims to produce a line of nutraceutical aquafeed additives as well as a bulk feed ingredient that can supplement fishmeal. Its process includes feeding carbon dioxide from industrial gas to a “microbial consortium” starring hydrogen-oxidizing bacteria.

Aquafeeds
Aquaculture Exchange: Rick Barrows
After 14 years with the USDA’s Agricultural Research Service, Rick Barrows talks about the importance of finding ‘complete’ and commercially viable alternative sources of omega-3 fatty acids and continuing innovation in the aquafeed sector.

Health & Welfare
Advances in tilapia nutrition, part 1
This two-part review brings together scientific and field advances in tilapia nutrition and feeding to better support the formulation of feeds for better production performance.

Intelligence
‘Quality map’ for coho salmon fillets identifies color, lipid distribution
In research on coho salmon fillets, muscle astaxanthin level, color, and hardness generally increased from the anterior toward the caudal zone of the fish.


