Highest mortality rates are found during the first 20 to 40 days of culture
Growing rapidly in recent decades, shrimp aquaculture is now one of the major global agriculture industries. In parallel, emerging shrimp diseases have also been on the rise, largely because shrimp are cultured at high density, exposed to environmental stress and traded globally.
Since 2009, early mortality syndrome, also termed acute hepatopancreatic necrosis disease (AHPND), has caused large-scale shrimp deaths and huge economic losses for shrimp farmers in China, Vietnam, Malaysia and Thailand. AHPND can affect Penaeus monodon, P. chinensis and Litopenaeus vannamei.
The highest mortality rates for shrimp with AHNPD are found during the first 20 to 40 days of culture in grow-out ponds. Typical signs of AHPND infection, which are found mainly in the digestive tracts of shrimp, include an empty gastrointestinal tract and abnormal shrunken, whitish hepatopancreas, which result in weakness and death in shrimp. AHPND infection is defined by histological changes in the hepatopancreatic system, particularly sloughing of hepatopancreas tubule epithelial cells.
In 2013, the causative agent of AHPND/EMS (AHPND V.ph) was identified as a unique pathogenic strain of Vibrio parahaemolyticus containing a virulence-associated plasmid. V. parahaemolyticus has been in marine environments worldwide.
Molecular identification
Recently, toxin 1, a homolog of an insecticidal toxin, has been shown to play a critical role in hepatopancreas damage caused by AHPND bacteria in diseased shrimp. Toxin 1 consists of two genes, pirA and pirB, and pirB alone could cause the typical histological changes associated with AHPND in shrimp hepatopancreas.
Virulence factor genes, including toxin 1, have been found within “pathogenic islands” flanked by inverted repeats of transposase genes in the so-called AHPND-associated plasmid. Therefore, both toxin 1 (pirA and pirB) and the AHPND-associated plasmid are potential markers to allow identification of virulent AHPND bacteria. The AHPND-associated plasmid could be identified by the presence of certain unique markers.
Various molecular methods targeting toxin 1 and/or the AHPND-associated plasmid are available to help APHND diagnosis. However, different Vibrio species readily exchange genetic materials among themselves through genetic transfer mechanisms, including homologous recombination, transposition, conjugation or transformation. Transposases can move genes horizontally from one location to another location.
So far, non-virulent V. parahaemolyticus strains containing truncated AHPND-associated plasmids with toxin 1 deletion have been found in the environment. Therefore, detection of toxin 1 alone is not recommended, since toxin 1 is located in an unstable region.
Although no evidence has been reported yet, it is suspected that the toxin 1 gene region could be transferred to another bacteria. And the new toxin 1-harboring bacteria might not be virulent, because AHPND pathogenesis may need other virulence factors.
On the other hand, it has been reported that 98 percent of the samples tested AHPND-associated plasmid positive by polymerase chain reaction were found to show AHPND symptoms. Therefore, detection of the plasmid alone could help detect a potential AHPND threat. Thus, to diagnose AHPND, detection of both the plasmid and toxin 1 gene is recommended.
When simultaneous performance of the two assays is not possible, due to cost concerns, for example, it is recommended to perform screening for the plasmid first to identify a potential threat. In case of plasmid-positive results, the toxin 1 assay could be used to provide further evidence of AHPND.
A good AHPND detection method also should not cross-react with host DNA, any irrelevant plasmids and other microorganisms. Because not much bioinformatics information is available for toxin 1 homologs, probe-based molecular methods should offer better specificity than conventional methods to detect targets of this nature.
On-site molecular detection tools
Without vaccines and effective treatments for shrimp diseases, the shrimp industry relies greatly on biosecurity measures to prevent the introduction of pathogens or risk factors to facilities to reduce the spread of pathogens throughout the culture environment.
Polymerase chain reaction (PCR) assays, with their high sensitivity and specificity, have been employed commonly at various large-scale aquaculture facilities for pathogen surveillance or disease diagnosis. This approach has proven to help detect pathogens at early stages to facilitate timely implementation of measures to control the spread of major shrimp disease agents such as white spot syndrome virus, improving overall shrimp production in the long run.
However, due to the challenging requirements for sophisticated equipment and highly trained personnel to perform PCR, small-scale farms in general have been reluctant to include PCR assays in their biosecurity measures. Therefore, user-friendly, field-deployable detection methods are now available to monitor and improve biosecurity at small-scale farms.
Optimal on-site detection systems should be rapid, inexpensive, sensitive and easy to maintain and perform for anyone with minimal training. In addition, the reagents should be provided in a format that allows easy shipping and storage.
Current on-site method
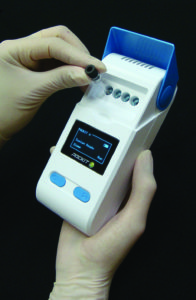
A molecular diagnostic assay based on insulated isothermal polymerase chain reaction (iiPCR) testing, working on a specific field-deployable device, is currently available for on-site detection of AHPND. The reliability of the iiPCR system has been demonstrated by a similar system certified by the World Organisation for Animal Health (OIE) as validated for detecting white spot syndrome virus in shrimp in 2013.
The portable device can amplify target sequence, detect fluorescent signals and display simple readouts within one hour. Two iiPCR assays, targeting either the most stable region of the AHPND plasmid or the virulence-related toxin 1, have been developed to monitor the markers in shrimp, water, live feed or other sources to help evaluate risks for AHPND.
The assays detect their targets sensitively and specifically. For example, the sensitivity of both iiPCR assays were comparable to that of real-time PCR assays, based on results of an endpoint dilution study using serial dilutions of a virulent AHPND V.ph strain (Table 1).
Chung, DNA dilutions, Table 1
| DNA Dilutions | toxin 1: Real-Time PCR | toxin 1: iiPCR Assay | AHPND Plasmid Real-Time PCR | AHPND Plasmid iiPCR Assay |
|---|---|---|---|---|
| -1 | 2/2 | 2/2 | 2/2 | 2/2 |
| -2 | 2/2 | 2/2 | 2/2 | 2/2 |
| -3 | 3/3 | 2/2 | 3/3 | 2/2 |
| -4 | 0/3 | 0/3 | 0/3 | 2/3 |
| -5 | 0/3 | 0/3 | 0/3 | 0/2 |
For specificity evaluation, cultures of 18 of the AHPND strains were tested. The toxin 1 iiPCR reacted positively with all eight toxin 1-positive strains and negatively with all 10 toxin 1-negative strains identified by a reference toxin-1 PCR assay from National Cheng Kung University in Tainan, Taiwan, indicating this reagent has excellent specificity in detecting the virulence marker of the AHPND V.ph.
Perspectives
Good diagnostic systems are mostly built on well-established technology and research results. Reagents should be manufactured under controlled environments with quality controls following international standard procedures and pass stringent validation procedures.
Application of these systems in pathogen detection can often be performed only on specific samples at specific time points. However, shrimp cultures generally involve huge shrimp populations associated with dynamic conditions over long periods of time. Therefore, to get the most of these tools, the users should also pay attention to sampling site, size and timing. Furthermore, for correct diagnosis of a disease, it is best to analyze the samples using multiple test methods based on different technologies, such as microbiology, histopathology and qualitative/quantitative PCR.
On the whole, bacteria such as the AHPND V.ph are hard to eliminate and could have long-term impacts on shrimp farms once they are introduced into the facilities. Screening and removal of potential AHPND threats from shrimp, water, live feeds or other sources is critical in biosecurity. On-site diagnosis should make the implementation of this important measure possible at shrimp culture facilities of different scales at any location.
(Editor’s Note: This article was originally published in the July/August 2015 print edition of the Global Aquaculture Advocate.)
Authors
-
Simon Chung
Vice President, Global Sales
GeneReach Biotechnology Corp.
19, Keyuan 2nd Road
Central Taiwan Science Park
Taichung 407 Taiwan -
Dr. Pei-Yu Alison Lee
Vice President, Research and Development
-
Li-Juan Ma
Director, Technical Service
GeneReach Biotechnology Corp.
Tagged With
Related Posts

Health & Welfare
Four AHPND strains identified on Latin American shrimp farms
Two virulence genes are known to encode a binary photorhabdus insect-related toxin that causes acute hepatopancreatic necrosis disease in shrimp. The pathogenicities of these V. campbellii strains were evaluated through laboratory infection and subsequent histological examination in P. vannamei shrimp.

Health & Welfare
Genetic variation for resistance to WSS, AHPND in Pacific white shrimp
Selection for disease resistance has been used in breeding farm animals and can be a viable option to deal with white spot syndrome and acute hepatopancreatic necrosis disease in commercial shrimp culture. In trials, heritability for AHPND resistance was low, while that for WSS was moderate.

Health & Welfare
PCR methods characterize AHPND V.p isolates
In analyzing the plasmid sequence from the whole genome sequences of AHPND V. parahaemolyticus (V.p) isolates, researchers identified a clear geographical variation within the plasmid, and developed PCR methods to characterize AHPND V.p isolates as either Mexico-type or Southeast Asia-type.

Health & Welfare
A holistic management approach to EMS
Early Mortality Syndrome has devastated farmed shrimp in Asia and Latin America. With better understanding of the pathogen and the development and improvement of novel strategies, shrimp farmers are now able to better manage the disease.


