Low-cost test could monitor passage of infection between wild, cultured shrimp
Necrotizing hepatopancreatitis bacterium (NHP-B), a pleomorphic, gram-negative rickettsial-like organism, has been responsible for significant losses of cultured shrimp in the Americas and could be a threat to other worldwide sources. It was originally identified in the U.S. state of Texas, but has now been documented in aquaculture farms throughout North, Central and South America. NHP-B infection is potentially treatable if it is detected early and medicated food is applied before the shrimp cease feeding due to lethargy and anorexia – symptoms of the disease.
Current tests for NHP, such as polymerase chain reaction (PCR) or histology, require sending samples to centralized laboratories, which can take several days and be costly. Pondside analysis of wet mounts for melanized tubules can lead to misdiagnosis due to early similarities to septic hepatopancreatic necrosis from vibriosis.
NHP is currently being considered for listing by the World Organisation for Animal Health as a reportable disease, which points to the urgent need for faster and more cost-effective methods of detection. The development of a non-invasive test using fecal samples from broodstock or extracts from hepatopancreas tissue from cultured shrimp would enable more rapid detection and monitoring of potential infections, and earlier intervention.
Test development
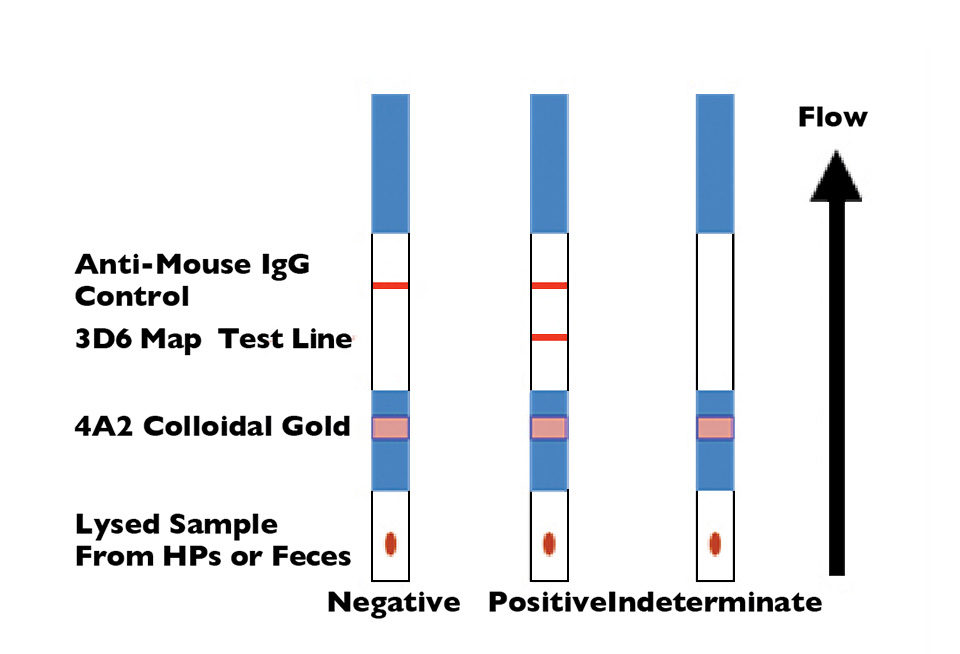
With research funding through the United States Department of Agriculture Cooperative State Research, Education and Extension Service, the authors have developed a rapid lateral-flow immunoassay using monoclonal antibody combinations specific to NHP-B that can be administered on site at the pond and provide results in less than 15 minutes. The principle of the assay is shown in Fig. 1.
The monoclonal antibodies are directed to an antigen detectable in native and denatured NHP-B, and shown to be present on all geographical isolates tested. Such a low-cost test would also have applications in monitoring the passage of the infection between wild and cultured shrimp.
Two monoclonal antibodies shown by western blot to detect a 13kDa component in the bacterium were used in the assay development. The target antigen is as yet unknown, but believed to be a carbohydrate moiety present in Percoll gradient fractions of hepatopancreas enriched by flagellar components of the NHP-B.
During a typical test with the dipstick method, hepatopancreas tissue and fecal samples were collected from Pacific white shrimp (Litopenaeus vannamei) experimentally infected with the NHP bacterium and extracted with a detergent containing lysis buffer. Dipstick reactivity was observed in both sample types when infection was detected by PCR or immunohistochemistry. Examples of these reactions are summarized in Table 1.
Houghton, Reactivity of PCR positive and negative hepatopancreas samples, Table 1
| Dipstick Rapid NHP-B Assay + | Dipstick Rapid NHP-B Assay – |
|---|
Dipstick Rapid NHP-B Assay + | Dipstick Rapid NHP-B Assay – | ||
|---|---|---|---|
| Polymerase | + | 10 | 3 |
| Chain Reaction | – | 0 | 33 |
Evaluations continue
The availability of this test can facilitate monitoring of NHP-B infection in the field and potentially reduce the significant production and revenue losses that are currently typical when infections of shrimp ponds with this organism occur. Ongoing field evaluations of the test will further determine its overall performance characteristics.
(Editor’s Note: This article was originally published in the July/August 2009 print edition of the Global Aquaculture Advocate.)
Now that you've finished reading the article ...
… we hope you’ll consider supporting our mission to document the evolution of the global aquaculture industry and share our vast network of contributors’ expansive knowledge every week.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year. GSA individual and corporate members receive complimentary access to a series of GOAL virtual events beginning in April. Join now.
Not a GSA member? Join us.
Authors
-
Raymond L. Houghton, Ph.D.
InBios International Inc.
562 First Avenue South, Suite 600
Seattle, Washington 98104 USA -
Jean Chen
InBios International Inc.
562 First Avenue South, Suite 600
Seattle, Washington 98104 USA -
Stan Morkowski, Ph.D.
InBios International Inc.
562 First Avenue South, Suite 600
Seattle, Washington 98104 USA -
Syamal Raychaudhuri, Ph.D.
InBios International Inc.
562 First Avenue South, Suite 600
Seattle, Washington 98104 USA -
Yvonne Stevens
InBios International Inc.
562 First Avenue South, Suite 600
Seattle, Washington 98104 USA -
Carlos Pantoja, Ph.D.
University of Arizona
Department of Veterinary Science and Microbiology
Tucson, Arizona, USA -
Bonnie T. Poulos
University of Arizona
Department of Veterinary Science and Microbiology
Tucson, Arizona, USA -

Donald V. Lightner, Ph.D.
University of Arizona
Department of Veterinary Science and Microbiology
Tucson, Arizona, USA
Tagged With
Related Posts

Health & Welfare
Updates on shrimp diseases AHPND, NHP at Aquaculture America 2018
Several shrimp-disease papers were presented at Aquaculture America 2018, including one on the detection of AHPND at a site in the United States.

Health & Welfare
Brazil shrimp farm performs genetic selection for IMNV resistance, growth
The Queiroz Galvão Alimentos shrimp farm and hatchery in Brazil have been working with Concepto Azul to implement a disease-prevention and genetic-breeding program that addresses ongoing impacts from infectious myonecrosis virus (IMNV) and other pathogens.

Health & Welfare
A holistic management approach to EMS
Early Mortality Syndrome has devastated farmed shrimp in Asia and Latin America. With better understanding of the pathogen and the development and improvement of novel strategies, shrimp farmers are now able to better manage the disease.

Health & Welfare
The unmet promise of pondside PCR
A new generation of technology, portable PCR, offers potential for affordable, immediate, pondside diagnosis in easy-to-use handheld kits. But will it live up to the hype?


