Acidic soils should be limed and pond bottoms should be dried out between crops

The pH and organic matter concentration of earthen pond bottoms are considered important by aquaculturists, and management techniques are often applied to raise pH and lower organic matter concentrations in bottom soil. Most aquaculturists, however, are less aware of how bottom soils and water interact to govern the basic composition of water in ponds.
Soil properties vary greatly from place to place, and water often is taken from sources that do not reflect the nature of soils in pond bottoms. There may be great differences among the basic, initial properties of pond soil and water. Watershed ponds are an exception, in that they are filled with runoff from the same watershed in which they are located.
Soil, water reactions
When water is added to a pond, it contacts the soil and various reactions occur. If the water is acidic and the bottom soil is basic, bases in the soil neutralize acidity in the water, raising its pH and total alkalinity. Of course, the opposite also occurs – alkaline water will neutralize acidic soil.
In cases where both soil and water are acidic, liming is used to neutralize soil acidity and assure adequate pH and total alkalinity in pond waters. Where both soil and water are basic in reaction, liming is unnecessary unless there are other sources of acidity. A common source of acidity in such aquaculture ponds is nitrification of ammonium from fertilizers or ammonia excreted by the culture species, and microbial decomposition of uneaten feed and feces. In some arid regions, alkaline soil and water result in undesirably high pH that usually cannot be mitigated.
Cation exchange
Cations attracted to negatively charged sites on clay and organic matter particles in soil can be exchanged with cations in water. The ability of a soil to adsorb cations by this process is called its cation exchange capacity (CEC) and is expressed in milliequivalents of cations per 100 grams of soil. Pond soils can have CECs of less than 5 to over 40 meq/100 grams.
Ion exchange occurs between soil and water until an equilibrium is reached. If cations are added to water at equilibrium, exchange will occur until a new equilibrium is obtained. Soils that contain expansible, layered clay minerals also can fix potassium and possibly other cations between the layers of the clay. Cations fixed in this manner are essentially unavailable to the water again.
Cation exchange and fixation can cause changes in concentrations of cations in pond waters. The best example of the aquaculture importance of these processes is in inland culture of marine shrimp in low-salinity water. Magnesium and potassium are removed from water through adsorption on cation exchange sites or by fixation of potassium by layered clay minerals. Periodic potassium and magnesium supplementation of waters in inland shrimp ponds often is necessary to avoid negative impacts of low concentrations of these two cations on shrimp survival and growth.
Soil minerals have some positive sites on which anions can be adsorbed. However, soil uptake of chloride, sulfate, bicarbonate, carbonate and nitrate by anion exchange is minor and does not influence the concentrations of these ions in water.
Soil minerals, groundwater
In semi-arid and arid regions, soils often contain soluble mineral salts that do not leach from the soil profile because of low rainfall relative to evaporation. When water is added to such ponds, these salts dissolve from the soil, increasing concentrations of major ions such as calcium, magnesium, sodium, potassium, sulfate, chloride and bicarbonate/carbonate.
If groundwater high in concentrations of calcium, total alkalinity and carbon dioxide is applied to a pond, the water equilibrates with carbon dioxide in the atmosphere and calcium carbonate precipitates. The calcium and alkalinity concentrations in the water decline without involvement of the soil, but the precipitating calcium carbonate becomes part of the soil.
Groundwater used to fill ponds may in some locations be high in total alkalinity but very low in calcium concentration. Such waters can develop extremely high pH as a result of photosynthesis. Reactions between soil and water seldom remedy the situation, because calcium is held more tightly to cation exchange sites in soil than sodium and potassium ions, which balance the electrical charge of the bicarbonate/carbonate ions in high-alkalinity, low-hardness water. This problem can usually be solved by applying calcium sulfate or other soluble calcium salt to increase calcium concentration in the water.
Soils have a large capacity to absorb phosphorus from water through reactions with iron and aluminum hydroxides, or with calcium in bottom soils. This process is illustrated in Fig. 1 by the rapid disappearance of phosphorus from pond water following fertilization.
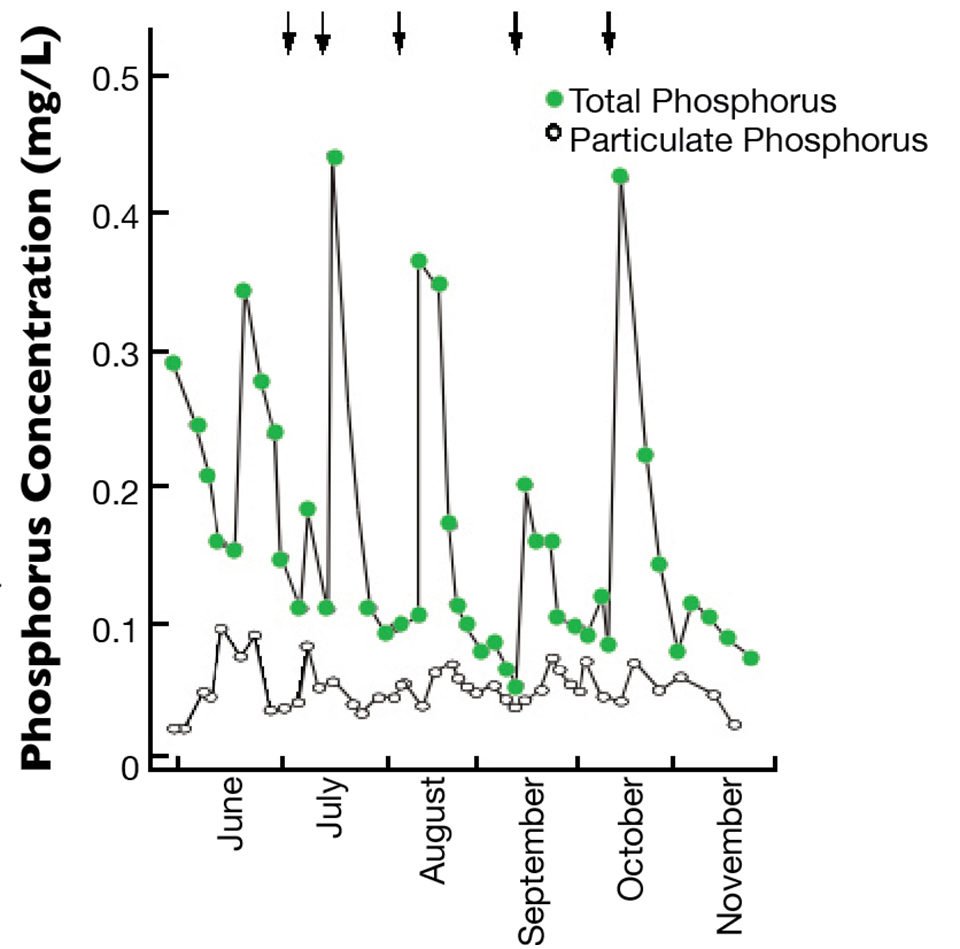
Once fixed in the soil, phosphorus is not highly available to the water column. Liming pond soils will increase pH and favor greater phosphorus solubility from compounds in the soil, but fertilizer still must be applied at frequent intervals to maintain a concentration sufficient for good phytoplankton growth. In ponds with high calcium concentrations, and especially if the pH is above 8, calcium phosphate will precipitate directly from the water and become part of the bottom soil.
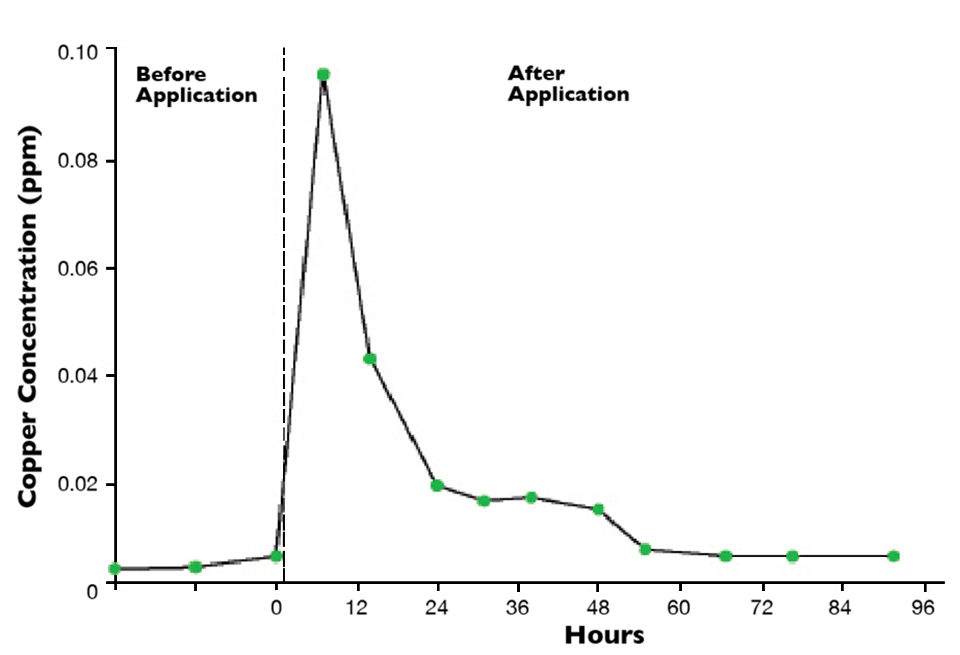
Bottom soil also adsorbs trace metals through cation exchange, but where pond waters are not acidic, trace metals tend to precipitate as highly insoluble minerals. For example, copper from copper sulfate used as an algicide in ponds can be adsorbed on cation exchange sites in bottom soil, but it also precipitates rapidly from water as copper oxide to enter the soil. The overall result is rapid loss of applied copper from water (Fig. 2).
Soil types that are likely to influence water quality are:
- strongly acidic soils – highly leached soils with low concentrations of major cations or soils containing iron pyrite that oxidizes to form sulfuric acid
- soils with a high cation exchange capacity of 20 meq/100 g or more, especially those that contain expansible, layered clay minerals
- soils with an organic carbon concentration above 3 mg/L
- soils that contain limestone, gypsum or other soluble minerals
- soils with a high clay concentration of 30 percent or more.
Water types that are likely to influence soil quality are:
- highly acidic water
- highly mineralized water
- water with a high concentration of substances that react with soil, such as phosphate or copper.
Microbial reactions
Pond bottoms also are a site of many microbial reactions. Organic matter from dead plankton, uneaten feed and feces settles to the bottom and becomes mixed with the soil. Soil has a large surface area and provides an excellent medium for decomposition of the organic matter, nutrient recycling and other microbial processes. In aerobic zones, the major products of organic matter decomposition are carbon dioxide, ammonia, phosphate, sulfate and other mineral elements.
The diffusion rate of oxygen is slow in soils, and microbes below the soil-water interface quickly use up dissolved oxygen in pore water. Under anaerobic conditions, microbial activity also releases nitrogen gas, nitrite, ferrous iron, manganeous manganese, sulfide, methane and partially reduced organic compounds. Some of these substances, nitrite and sulfide in particular, can be highly toxic to fish and shrimp.
It is important to manage ponds in a manner that reduces the likelihood of the anaerobic zone extending to the soil-water interface. Practices that improve bottom soil conditions include the prevention of overfeeding and adequate mechanical aeration to maintain dissolved oxygen concentrations above 3 mg/L and circulate water across the pond bottom.
Acidic soils should be limed, and pond bottoms should be dried out between crops to encourage decomposition of organic matter and oxidize reduced inorganic compounds. Measures to control erosion in ponds should be implemented to lessen the accumulation of soft, organically enriched sediment.
(Editor’s Note: This article was originally published in the May/June 2011 print edition of the Global Aquaculture Advocate.)
Authors
-

Claude E. Boyd, Ph.D.
Department of Fisheries and Allied Aquacultures
Auburn University
Auburn, Alabama 36849 USA -
Li Li
Department of Fisheries and Allied Aquacultures
Auburn University
Auburn, Alabama 36849 USA
Tagged With
Related Posts

Health & Welfare
AHPN inferences based on behavior of vibrio bacteria
Vibrio parahaemolyticus, a strain of which is the cause of acute hepatopancreatic necrosis (AHPN), has both virulent and benign strains. This strain colonizes the stomachs of shrimp by the formation of a biofilm, which protects it from antibiotics and other potential treatments.

Innovation & Investment
Acoustic control improves feeding productivity at shrimp farms
In systems recently developed for shrimp farms, passive acoustic-based technology enables sensor-based control of multiple automatic feeders. Improved growth and feed conversion have been recorded at commercial farms using the technology.

Responsibility
Addressing safety in Latin America’s tilapia supply chain
Over the last decade, the experience gained by many tilapia farmers combined with proficient programs implemented by local governments have significantly improved tilapia production in various Latin American countries like Colombia, Mexico, Ecuador and other important tilapia producers in the region.

Health & Welfare
Ammonia toxicity degrades animal health, growth
Ammonia nitrogen occurs in aquaculture systems as a waste product of protein metabolism by aquatic animals and degradation of organic matter, or in nitrogen fertilizers. Exposure can reduce growth and increase susceptibility to diseases in aquatic species.


